Controllable electrophoresis deposition of TiO2 mesoporous spheres onto Ti threads as photoanodes for fiber-shaped dye-sensitized solar cells
Abstract
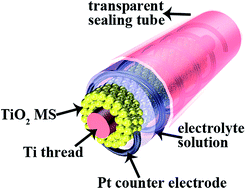
Complex TiO2 mesoporous spheres (MSs) were deposited on a Ti thread using an electrophoresis deposition (EPD) technique as working electrodes of the fiber-shaped dye-sensitized solar cell (FDSSC). The thickness of the TiO2 MS film can be controlled by altering EPD time in a constant voltage. Electrochemical impedance spectroscopic measurement demonstrates the film thickness-dependent photovoltage performance of the FDSSC. The total conversion efficiency of the FDSSC achieves 3.8% through optimizing the thickness of the film.

 Please wait while we load your content...
Please wait while we load your content...