Deploying clinical grade magnetic nanoparticles with magnetic fields to magnetolabel neural stem cells in adherent versus suspension cultures
D. Weinbergab,
C. F. Adamsa and
D. M. Chari*ab
aCellular and Neural Engineering Group, Institute for Science and Technology in Medicine, Keele University, Keele, Staffordshire, ST5 5BG, UK. E-mail: d.chari@keele.ac.uk; Tel: +44 (0)1782733314
bSchool of Medicine, Keele University, David Weatherall Building, Staffordshire ST55BG, UK
First published on 30th April 2015
Abstract
Neural stem cells (NSCs) have a high therapeutic potential for patients with neurological disease/injury given their neuroregenerative and immunomodulatory capabilities. In recent years, magnetic nanoparticles (MNPs) have been used as contrast agents in translational studies, to track transplanted NSCs using non-invasive magnetic resonance imaging (MRI). However, NSC uptake of MNPs is inherently low in the absence of chemical/biological uptake enhancing strategies such as cell targeting peptides and transfection agents – approaches which may be cytotoxic and alter cellular physiology. By contrast, physical delivery strategies relying on magnetic assistive methods can safely enhance MNP uptake into multiple neural cell types. The utility of this approach has been demonstrated for gene delivery grade particles but their application in enhancing ‘magnetolabelling’ with clinical grade contrast agents has never been evaluated. Here, we show that applied oscillating magnetic fields can safely enhance the uptake of a clinical grade MNP (Lumirem/Ferumoxsil) into NSCs propagated as neurospheres (suspension cultures, the preferred format for transplantation) but offer limited benefit for monolayer (adherent) cultures. This physical delivery method therefore has potential to facilitate cell labelling for clinical therapies.
Introduction
Neurodegenerative diseases and injuries are highly debilitating, with current treatment options limited largely to offering symptomatic relief as opposed to disease-modifying benefits.1 Neural stem cell (NSC) transplant populations offer important therapeutic potential for such patients, mediating repair in multiple pre-clinical disease models including Parkinson's disease, multiple sclerosis and stroke1,2 with multiple clinical trials underway. NeuralStem (USA) is currently undertaking a phase I clinical trial (NCT01348451) for amyotrophic lateral sclerosis. The therapy was deemed safe,3 and NeuralStem have since initiated a phase II clinical trial in May 2013 (NCT01730716). ReNeuron (UK) is investigating the transplantation of NSCs in patients with ischaemic stroke, recently launching a phase II clinical trial (NCT02117635) at 10 UK centres. The ‘homing’ ability of NSCs towards sites of inflammation and pathology (termed ‘pathotropism’)4,5 is being exploited in patients with recurrent high-grade gliomas at City of Hope Medical Center (California, USA), utilising NSCs expressing the pro-drug activating enzyme cytosine deaminase to induce tumour shrinkage (NCT01172964).6 With regard to NSC transplantation for such patients, an important limitation remains – namely the need for safe, real time in vivo tracking methods to correlate delivery of therapeutic NSCs with neurological outcomes.7 Such information can facilitate successful cell delivery and provide valuable prognostic information with regard to cell proliferation and migration.8,9 Additionally, the migratory efficacy of MNP-labelled NSCs towards lesions may differ between delivery routes, hence information on cell biodistribution could be utilised to determine optimal delivery routes and cell retention at sites of pathology.7Magnetic nanoparticles (MNPs) are evolving as advanced materials for multiple biomedical applications including gene and drug delivery,10 magnetic hyperthermia,11 magnetic manipulation and targeting,12 and cell tracking.7 The iron oxide core of MNPs (usually magnetite or maghemite)10 enables tracking of MNP-labelled cells using magnetic resonance imaging (MRI), which is clinically advantageous due to its non-invasiveness, high spatial resolution and absence of ionising radiation.7 Further, MRI displays oedematous and inflammatory pathology to provide important clinical prognostic information.7 A major translational barrier however is that MNP uptake into NSCs is inherently low (10–20%)13 without increasing MNP concentrations/incubation times or the use of biochemical adjuncts including cell-penetrating peptides and transfection agents (TAs). These can alter cell physiology and TAs carry a significant toxicity risk and can cause MNP agglomeration.9,14 This is problematic for cell tracking as membrane adherent MNP aggregates may become detached post-transplantation, with inaccurate representations of transplant distribution.14 As a further example, heparin and protamine are two clinically-approved transfection agents widely used to enhance ferumoxytol uptake into cells.15,16 The heparin constituent of this so-called ‘HPF complex’ may potentially be hazardous in causing microhaemorrhage following breakdown of complexes. Furthermore, this could disrupt haemostasis mechanisms following transplantation and potentially lead to local haemorrhage, of concern from a clinical perspective.17 A potential solution to these translational challenges is a recent innovation in nanotechnology using magnetic assistive technology (applied static/oscillating magnetic fields) to safely enhance cellular MNP uptake. Magnetic assistive methods offer multiple advantages for translational neurology and transplant populations. They have been shown to be safe18–21 as they exploit endogenous endocytic machinery of cells and leave minimal residual toxic effects following transplantation, as opposed to the use of TAs. These methods are also relatively straightforward compared to other biochemical strategies, simply necessitating application of MNPs to cells in the presence of a magnetic field.
We have previously proved that oscillating magnetic fields can enhance uptake of transfection grade MNPs into major neural cell types including NSCs,20 oligodendrocyte precursor cells18 and astrocytes22 and enhance uptake of polymeric MNPs in NSCs.23 However, to our knowledge, the utility of this approach has never been evaluated for uptake of clinical grade MNP contrast agents into neural transplant populations, specifically NSCs. It is important to address this issue, as the efficacy of magnetic assistive methods with MNPs is dependent on a range of physicochemical parameters including particle size/density, Fe content and fluid viscosity.23 Particle uptake by cells also depends on several parameters such as particle aggregation, size, surface chemistry and charge. As transfection/contrast grade MNPs show considerable heterogeneity in their physicochemical properties, it is essential to systematically investigate the efficacy of magnetic assistive technology on a particle by particle basis but little information currently exists on the influence of MNP chemistry on uptake into neural cells. Here, we have investigated the effects of oscillating magnetic fields on uptake of a clinical grade contrast agent (Lumirem/Ferumoxsil) into NSCs. Lumirem is clinically approved as a contrast agent for bowel imaging and contains siloxane-coated superparamagnetic iron oxide nanoparticles (SPIONs) with a reported hydrodynamic diameter of 300 nm.24 The siloxane coating is poorly soluble, protecting the iron oxide core from degradation and potential cytotoxicity.25
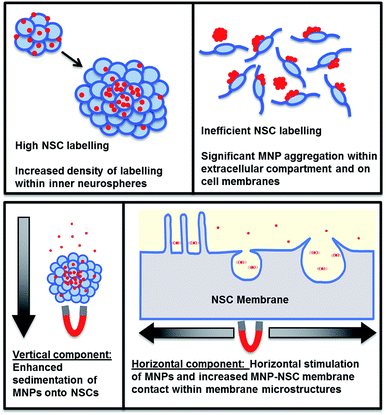
It is important to note here that NSCs are propagated by two well characterised and globally used culture formats – adherent ‘monolayers’ and suspension ‘neurospheres’, both offering distinct advantages for cell labelling and transplantation (summarised in Fig. 1).26 For example, transplantation of neurospheres is thought to result in higher survival rates than single cells.27 However, monolayer culture allows all the cells to be exposed to particles, potentially facilitating greater particle uptake throughout the cell population when compared to neurosphere culture (which contains ‘hidden’ cells inside the sphere). As either system may be used to culture human NSCs for clinical application,28 it is imperative that magnetolabelling procedures are evaluated between the two culture formats, for translational studies. However, to the best of our knowledge, such a comparative labelling analysis has not been conducted previously. Given these knowledge gaps, the study goals were as follows (i) to determine whether applied oscillating magnetic fields can enhance Lumirem uptake in primary NSCs; (ii) to establish whether this combined approach is safe for NSCs; (iii) to evaluate the influence of NSC culture format on labelling efficacy by directly comparing Lumirem uptake in monolayers versus neurospheres.
 | ||
| Fig. 1 Schematic diagram outlining the advantages/disadvantages of monolayer and neurosphere culture formats for NSCs. | ||
Materials and methods
Reagents
Thermo scientific Nunc culture plates (non-treated surface) and other cell culture grade plastics were purchased from Fisher Scientific, UK. Cell culture reagents were purchased from Life Technologies (Paisley, Scotland, UK) and Sigma (Poole, Dorset, UK). Human recombinant basic fibroblast growth factor (bFGF) was also from Sigma and human recombinant epidermal growth factor (EGF) from R&D Systems Europe Ltd (Abingdon, UK). Penicillin and streptomycin were from Fisher (Loughborough, UK). Accutase was from Sigma and DNaseI was from Roche (Welwyn, UK).Lumirem was purchased from Guerbet (Solihull, UK). The magnefect-nano oscillating magnetic array system, used for oscillating magnetofection methods, was from nanoTherics Ltd. (Stoke-on-Trent, UK; commercially available since 2009). This system allows culture plates to be placed over a horizontal array of neodymium (NdFeB) magnets, grade N42, which match the plate configuration. The magnetic array can be programmed to oscillate laterally beneath the culture plate via a computerized motor; both the frequency (up to 5 Hz) and the amplitude (10 μm to 1 mm) of oscillation can be varied. The field strength at the face of each magnet is 421 ± 20 mT (nanoTherics Ltd., personal communication). In static mode (frequency, F = 0 Hz), this system has been shown to produce similar transfection efficiencies to commercially available magnetic plates in routine use for static magnetofection and (to our knowledge) it is the only commercially available device for oscillating magnetofection applications.
Primary antibodies were for nestin (a NSC cytoskeletal protein) from BD Biosciences (Oxford, UK), sox-2 (a NSC transcription factor) from Millipore (Watford, UK), β-tubulin (TUJ-1, detects neurons) from Covance (Princeton, NJ, USA), glial fibrillary acidic protein (GFAP, detects astrocytes) from DakoCytomation (Ely, UK), and myelin basic protein (MBP, detects oligodendrocytes) from Serotec (Kidlington, UK). FITC-conjugated secondary antibodies were from Jackson Immunoresearch Laboratories Ltd (West Grove, PA, USA). Chemicals for Perls' stain were purchased from Sigma.
The care and use of all animals used for cell culture were in accordance with the Animals Scientific Procedures Act of 1986 (UK) and following local ethical approval.
MNP characterisation
When exposed to culture medium, MNP physicochemical properties (such as size and surface chemistry) can change due to particle aggregation and formation of a ‘protein corona’ around the particle.29 As both size and surface charge could affect particle interaction with the cellular membrane and, ultimately, cellular uptake, the hydrodynamic diameter and zeta potential of Lumirem (which has not been fully characterised previously) were measured. This was performed in both PBS and monolayer medium (ML-M) in which NSCs are propagated, using a Zetasizer Nano ZS (Malvern, UK). Briefly, MNPs were added at a concentration of 10 μg ml−1 iron to either PBS or ML-M and left to incubate at 37 °C, 5% CO2 (24 hour samples) or added just prior to Zetasizer measurements in pre-incubated media (5 minute samples). To further assess particle size and shape, particles were placed in pure water, air-dried onto aluminum stubs, and observed uncoated using a high-resolution field emission scanning electron microscope (Hitachi S4500) operated at an accelerating voltage of 5 kV.NSC culture
NSCs were maintained and selectively expanded in suspension under stimulation from growth factors, to form neurospheres. Briefly, NSCs were derived from the subventricular zone of postnatal day 1–3 CD1 mice. Following isolation, NSCs were maintained in neurosphere medium (NS-M) comprising a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mix of DMEM
1 mix of DMEM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) F12 containing 2% B-27 supplement, 50 U ml−1 penicillin, 50 mg ml−1 streptomycin, 4 ng ml−1 heparin, 20 ng ml−1 bFGF and 20 ng ml−1 EGF. NSCs were fed every 2–3 days and neurospheres were passaged every 5–7 days by dissociation, both mechanically and using an accutase-DNaseI mix. For neurosphere labelling, NSCs (passages 1–3) were dissociated and plated in suspension at a cell density of 5 × 104 cells per ml (500 μl per well) using NS-M in Nunc 24 well non-treated culture plates. For labelling NSCs as monolayers, NSCs were re-plated in Nunc Nunclon Delta 24 well plates at a density of 7 × 104 cells per ml (600 μl per well) using ML-M comprising a 1
F12 containing 2% B-27 supplement, 50 U ml−1 penicillin, 50 mg ml−1 streptomycin, 4 ng ml−1 heparin, 20 ng ml−1 bFGF and 20 ng ml−1 EGF. NSCs were fed every 2–3 days and neurospheres were passaged every 5–7 days by dissociation, both mechanically and using an accutase-DNaseI mix. For neurosphere labelling, NSCs (passages 1–3) were dissociated and plated in suspension at a cell density of 5 × 104 cells per ml (500 μl per well) using NS-M in Nunc 24 well non-treated culture plates. For labelling NSCs as monolayers, NSCs were re-plated in Nunc Nunclon Delta 24 well plates at a density of 7 × 104 cells per ml (600 μl per well) using ML-M comprising a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mix of DMEM
1 mix of DMEM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) F12, containing 1% N2 supplement, 10 ng ml−1 bFGF and 10 ng ml−1 EGF with above antibiotic and heparin concentrations. NSCs in both culture formats were plated for 24 h prior to Lumirem addition.
F12, containing 1% N2 supplement, 10 ng ml−1 bFGF and 10 ng ml−1 EGF with above antibiotic and heparin concentrations. NSCs in both culture formats were plated for 24 h prior to Lumirem addition.
MNP incubation protocol
A range of concentrations (2–32 μg ml−1 iron) were initially trialled for labelling of NSCs in both monolayer and neurosphere culture formats. Concentrations of 2–8 μg ml−1 iron resulted in inefficient levels of uptake and those above 12 μg ml−1 iron led to loss of NSC adherence and cytotoxicity, determined by the presence of pyknotic nuclei and cell loss. Hence, a concentration of 10 μg ml−1 iron was used for final experiments. Lumirem MNPs were added in a drop-wise fashion to neurospheres whilst gently swirling the culture plate. For monolayers, Lumirem MNPs were added to NSCs in fresh ML-M. For each culture system, plates contained wells with treated and untreated cells (controls) and were incubated under no-field or magnetic field application. Plates undergoing oscillating magnetic field stimulation were added to the oscillating magnefect-nano device immediately following MNP addition for 30 minutes (frequency = 4 Hz). This frequency was adopted for experiments as it has previously been shown to be optimal for transfection of neurospheres with transfection-grade MNPs; static fields were not used in these experiments as these have been shown to be without effect on neurospheres.19,20 Cells were incubated at 37 °C, 5% CO2 for 48 hours prior to carrying out microscopic analyses.Neurosphere dissociation and plating as monolayers
Following 48 hours incubation of neurospheres with or without Lumirem, neurospheres under different treatment/magnetic field conditions were dissociated as described above, and re-plated as 2D adherent monolayers onto coverslips coated with poly-L-ornithine and laminin. NSCs were left to adhere overnight for 18 hours before fixing for subsequent staining and analysis. For NSCs undergoing differentiation, dissociated cells were re-plated as described above, using differentiation medium (NS-M without growth factors, supplemented with 0.5% fetal bovine serum). NSCs were differentiated for 5–7 days with 50% medium changes every 2–3 days.Immunocytochemistry and Perls' staining
NSCs labelled as neurospheres were fixed at 18 hours following dissociation, and those labelled as monolayers were fixed at 48 hours following MNP incubation. NSCs were fixed using 4% paraformaldehyde for 15 minutes at room temperature (RT) and then washed three times with PBS. For analysis of Lumirem uptake in NSCs, cells were stained with Perls' Prussian Blue (20 minutes, RT). Perls' stain was prepared by mixing equal volumes of 4% HCl with 4% potassium ferrocyanide. Stained cells were washed twice with PBS, counterstained with 1% neutral red before dehydration (30 seconds in 100% ethanol followed by 30 seconds each in two xylene washes) and mounting using DPX. For immunocytochemistry, coverslips were blocked (5% normal donkey serum in PBS–0.3% Triton–X-100) for 30 minutes at RT. Primary antibodies were diluted in blocking solution as follows before addition to cells: nestin 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 (NSCs), sox-2 1
200 (NSCs), sox-2 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 (NSCs), TUJ-1 1
100 (NSCs), TUJ-1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 (neurons), GFAP 1
1000 (neurons), GFAP 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 (astrocytes), MBP 1
500 (astrocytes), MBP 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 (oligodendrocytes). Following overnight incubation at 4 °C, cells were washed with PBS and blocked (30 minutes, RT). Secondary FITC-conjugated antibodies were added in blocking solution (1
200 (oligodendrocytes). Following overnight incubation at 4 °C, cells were washed with PBS and blocked (30 minutes, RT). Secondary FITC-conjugated antibodies were added in blocking solution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200) for 2 hours (RT), and then washed with PBS. Immunostained samples were mounted with Vectashield mounting medium containing DAPI.
200) for 2 hours (RT), and then washed with PBS. Immunostained samples were mounted with Vectashield mounting medium containing DAPI.
Microscopy and labelling analysis
The safety of labelling NSCs with Lumirem was assessed in two ways: (i) following Lumirem incubation with neurospheres, (ii) post-fixation of dissociated NSCs plated as monolayers. Phase-contrast microscopy of live neurospheres was performed following 48 hours Lumirem incubation prior to neurosphere dissociation using a Leica DM IL LED inverted microscope (Leica Microsystems, Wetzlar, Germany). Two fields were captured from four separate wells using a FC420C digital camera and Leica Applications Suite software (version 3.4.0) and then analysed in ImageJ for neurosphere counts per field and neurosphere size. Neurospheres with a diameter of <30 μm were excluded from these analyses as these were immature neurospheres, and generally difficult to discriminate as clusters of NSCs as opposed to cellular debris.Perl's and immunostained samples were imaged using an Axio Scope A1 fluorescence microscope (Carl Zeiss Microimaging, Germany). Perls' stained and DPX mounted NSCs were imaged at 100× magnification and these images used for analysis in ImageJ. Analyses of Lumirem uptake were performed on >35 cells over three fields from three replicate coverslips. Images of immunostained NSCs and daughter cell types were also analysed in ImageJ to assess NSC ‘stemness’ and differentiation potential respectively. Here, >100 DAPI-stained nuclei were assessed over three replicate coverslips for all analyses. These images were used to carry out cytotoxicity analyses by counting pyknotic (nuclear shrinkage, fragmented chromatin) DAPI nuclei for each condition.
Statistical analysis
All data were analysed using GraphPad Prism statistical analysis software (version 6.0) and are expressed as mean ± SEM unless stated otherwise. The number of experiments (n) refers to the number of NSC cultures, each derived from a different litter.Results
MNP characterisation
Zetasizer measurements of the particles in PBS and ML-M revealed a mean size of about 560 nm and no significant aggregation, or change in surface charge, of the particles over 24 hours (Fig. 2A). A small increase was observed at 5 minutes in ML-MM, however at the longer time point of 24 hours, no significant particle aggregation could be noted for particles in ML-MM compared with PBS. SEM of particles revealed a spherical shape and confirmed they were within the size range measured by dynamic light scattering (DLS), with some variation in size apparent (Fig. 2B).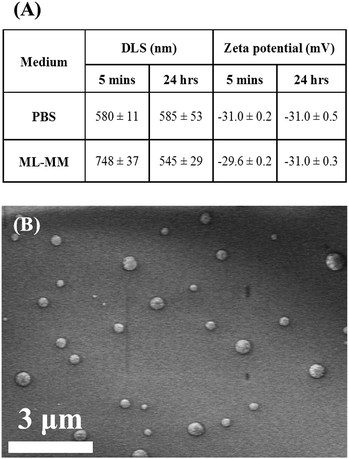 | ||
| Fig. 2 (A) Summary of Lumirem MNP zetasizer characterization (mean ± SD). (B) SEM micrograph of Lumirem particles. | ||
Lumirem labelling of NSCs as monolayers is ineffective
After adding MNPs to NSCs cultured as monolayers, extensive aggregation of MNPs and the formation of chain-like structures were noted (Fig. 3A, inset). This was evident when NSCs were labelled under stimulation by the oscillating magnetic field. There was little evidence of MNP uptake into cells. Further, marked cell loss was apparent in these experiments, and the remaining adherent cells frequently displayed abnormal cellular morphologies (evidence of cellular rounding up, shrinkage or shortened cell processes compared to control) (Fig. 3A).Lumirem uptake into neurospheres is enhanced by applied oscillating magnetic fields
For neurospheres, incubation with Lumirem resulted in 100% of neurospheres displaying some labelling, which was denser within the central two-thirds of the spheres (Fig. 3B). Compared with the no field condition, neurospheres labelled under the 4 Hz field appeared more densely labelled (Fig. 3B and C); therefore, spheres were dissociated in order to assess the numbers of labelled cells in each condition. After dissociation, the percentage of cells displaying Perls' staining was significantly higher when derived from spheres treated under the 4 Hz condition (54.4 ± 7.1%) compared to the no field condition (35.2 ± 4.6%) (Fig. 3D), with cells derived from the 4 Hz condition appearing to show larger intracellular particle accumulations as well (Fig. 3B, inset).Lumirem labelling of neurospheres is safe and does not affect neurosphere growth
Based on the results from the labelling study, which show evidence of Lumirem toxicity in NSCs cultured as monolayers, safety assays were performed only on the neurosphere population after labelling. No significant differences were found in neurosphere counts per unit area (Fig. 4A) or size (Fig. 4B) across treatment and field conditions, indicating that Lumirem treatment did not affect the proliferative capacity of NSCs. Cytotoxicity analyses of pyknotic nuclei revealed no significant differences between Lumirem-treated and untreated (control) NSCs, suggesting Lumirem added at an iron concentration of 10 μg ml−1 is non-toxic to NSCs labelled as neurospheres. Further, pyknotic nuclei counts, indicative of dying cells, were low across all conditions with a range from 2–5% (Fig. 4C). NSCs were >95% positive for nestin and 100% positive for sox-2, indicating that NSC stemness was not affected (Fig. 4D, E and G).The differentiation potential of NSCs is unaffected by Lumirem labelling
Following differentiation, no significant differences were found in the proportions of neurons, astrocytes or oligodendrocytes generated between Lumirem-treated and untreated (control) NSCs (Fig. 4F, H–J), indicating that Lumirem labelling does not affect NSC differentiation fate.Discussion
For the first time, we have performed a direct comparison between two globally used NSC culture formats to assess the use of oscillating magnetic assistive technology on the uptake of an FDA approved clinical-grade MNP. The clinical use of Lumirem, its superparamagnetic properties and the lack of literature investigating its uptake in neural cell types led us to investigate this MNP in the current study. Lumirem uptake has only been studied in retinal pigment epithelial (ARPE19) cells for translational purposes in retinal diseases so far; the cells were cultured as monolayers however no field application was used in this study.30 The use of primary NSCs in our study, as opposed to cell lines, increases the biological relevance and validity of the results as cell lines tend to behave in a homogenous and clonal manner and are typically immortal having undergone many passages. They also show resistance to cell death, reducing their utility for translational studies.31 Our findings demonstrate that applied oscillating magnetic fields safely enhance particle uptake in a major neural transplant population propagated as suspension (but not adherent) cells, with no adverse effects on key regenerative properties of the labelled cells.Neurospheres offer multiple advantages from a transplantation perspective (summarised in Fig. 1). First, neurospheres contain a high cell density within a small surface area, meaning greater numbers of NSCs can be labelled without the use of extensive culture equipment.20 Second, neurospheres have greater survival rates post-transplantation,27 vital for successful cell therapy. Neurospheres are also technically easy to culture, circumventing issues related to appropriate cell density and adherence encountered with the monolayer culture format.
The mechanism by which the oscillating magnetic field enhances Lumirem labelling of NSCs is poorly understood. A combination of enhanced sphere sedimentation in conjunction with MNP-membrane stimulation by oscillating fields may offer a reasonable explanation (Fig. 5). Interestingly, the inner two thirds of neurospheres were more densely labelled compared to the periphery, which may be explained by neurosphere biology. MNPs became associated with small neurospheres at 24 hours following incubation. These cells, which form the central portion of the neurosphere are more highly phagocytic of apoptotic cells.32 Additionally, NSCs towards the periphery of neurospheres display greater mitotic activity32 explaining the lower density labelling towards peripheries. Therefore, for clinical translation of this labelling method to be successful, neurosphere sizes would have to be controlled prior to transplantation procedures, in order to achieve optimal labelling efficiency.
From the MNP aggregation and loss of cell viability observed following MNP and field application to adherent cells, we conclude that a monolayer culture format is not appropriate for Lumirem labelling of NSCs. The underlying basis for these effects is currently unclear. This could be explained by either a physical disruptive mechanism or an effect on NSC surface membranes. The former is evident by the loss of NSC adherence, which was noted to be increased by the presence of an oscillating magnetic field. Here, the chain-like formations of Lumirem MNPs may cause high mechanical stress on NSC membranes and consequent loss of adherence, which is further exacerbated by stimulation under an oscillating magnetic field. Additionally, the effect of MNP chains coating NSC membrane surfaces may impede the uptake of smaller MNP aggregates by creating a physical barrier. We have not been able to find any other studies investigating the influence of a monolayer format on magnetolabelling with MNPs, and further investigations in this regard would yield interesting data. By contrast, it should be noted that transfection grade MNPs yield highly effective transfection in monolayers compared with neurospheres,33 highlighting the need to study variations in the uptake of different MNP classes in neural cells, for tissue engineering applications. Although oscillating fields enhance particle uptake in neurospheres, multiple strategies could be employed to further enhance labelling efficacy – employed either independently or in combination depending on their safety and efficacy. These could include: increased particle concentrations and incubation times (Table 1); integrated methods for cell separation and particle labelling such as that used by the ‘Magselectofection’34 technique; and use of novel magnetic assistive devices with a range of oscillation formats, with testing of their imaging potential. A systematic study is also required to test neural transplant cell labelling with a range of clinical contrast agents, to identify those most compatible with magnetic assistive technology, whilst offering acceptable safety profiles.
| Cell type | MNP | Particle formulation | Concentration | Time | Results | Reference |
|---|---|---|---|---|---|---|
| HB1F3 hNSCs | Feridex | Dextran | 25 μg ml−1 | 24 h | 0.04 ± 0.01 pg iron/cell | 35 |
| Primary rat NSCs | Feridex | Dextran | 75 μg ml−1 | 48 h | 5.30 ± 1.10 pg iron/cell | 14 |
| Primary human NSCs | RAFT diblock coated USPIOs | 95% methoxy(poly-ethylene glycol), 5% Rhodamine B end-functionalised polyacrylamide | 10 μg ml−1 | 2 h | 0.58 ± 0.08 pg iron/cell | 36 |
| 10 μg ml−1 | 24 h | 0.91 ± 0.04 pg iron/cell | ||||
| 100 μg ml−1 | 2 h | 1.68 ± 0.14 pg iron/cell | ||||
| 100 μg ml−1 | 24 h | 2.90 ± 0.60 pg iron/cell | ||||
| Human NSC cell line | Endorem | Dextran | 12.5 μg ml−1 | 24 h | ≈20% cells labelled | 13 |
| 100 μg ml−1 | 24 h | >95% cells labelled | ||||
| Sinerem | Dextran | 100 μg ml−1 | 24 h | <10% cells labelled | ||
| 800 μg ml−1 | 24 h | ≈65% cells labelled |
Conclusions
In summary, we have demonstrated that applied oscillating magnetic fields can be used to safely enhance the uptake of Lumirem, a clinical grade MNP, into NSCs propagated as neurospheres, for MRI tracking purposes following transplantation. By contrast, we conclude that the monolayer culture format is not compatible with this labelling approach, highlighting the important influence of cell culture growth method on the efficacy of magnetic assistive technology deployed with clinical grade MNPs.Acknowledgements
CFA was supported by the EPSRC Doctoral Training Centre in Regenerative Medicine and currently holds an EPSRC E-TERM Landscape Fellowship. DW is supported via the Keele Medical Intercalated Degree programme. DW and CFA contributed equally to this manuscript.Notes and references
- A. Tsukamoto, N. Uchida, A. Capela, T. Gorba and S. Huhn, Stem Cell Res. Ther., 2013, 4, 102 CrossRef PubMed.
- G. Martino and S. Pluchino, Nat. Rev. Neurosci., 2006, 7, 395–407 CrossRef CAS PubMed.
- J. D. Glass, N. M. Boulis, K. Johe, S. B. Rutkove, T. Federici, M. Polak, C. Kelly and E. L. Feldman, Stem Cells, 2012, 30, 1144–1151 CrossRef CAS PubMed.
- F.J. Müller, E. Y. Snyder and J. F. Loring, Nat. Rev. Neurosci., 2006, 7, 75–84 CrossRef PubMed.
- M. C. Oh and D. A. Lim, Neurotherapeutics, 2009, 6, 458–464 CrossRef CAS PubMed.
- K. S. Aboody, J. Najbauer, M. Z. Metz, M. D'Apuzzo, M. Gutova, A. J. Annala, T. W. Synold, L. A. Couture, S. Blanchard, R. A. Moats, E. Garcia, S. Aramburo, V. V. Valenzuela, R. T. Frank, M. E. Barish, C. E. Brown, S. U. Kim, B. Badie and J. Portnow, Sci. Transl. Med., 2013, 5, 184ra59 Search PubMed.
- S. M. Cromer Berman, P. Walczak and J. W. M. Bulte, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2011, 3, 343–355 CrossRef PubMed.
- J. W. M. Bulte, I. D. Duncan and J. A. Frank, J. Cereb. Blood Flow Metab., 2002, 22, 899–907 CrossRef PubMed.
- L. Li, W. Jiang, K. Luo, H. Song, F. Lan, Y. Wu and Z. Gu, Theranostics, 2013, 3, 595–615 CrossRef PubMed.
- S. C. McBain, H. H. Yiu and J. Dobson, Int. J. Nanomed., 2008, 3, 169–180 CAS.
- A. Singh and S. K. Sahoo, Drug Discovery Today, 2013, 19, 474–481 CrossRef PubMed.
- V. Vaněček, V. Zablotskii, S. Forostyak, J. Růřička, V. Herynek, M. Babič, P. Jendelová, S. Kubinová, A. Dejneka and E. Syková, Int. J. Nanomed., 2012, 7, 3719–3730 CrossRef PubMed.
- M. Neri, C. Maderna, C. Cavazzin, V. Deidda-Vigoriti, L. S. Politi, G. Scotti, P. Marzola, A. Sbarbati, A. L. Vescovi and A. Gritti, Stem Cells, 2008, 26, 505–516 CrossRef CAS PubMed.
- C.-C. V. Chen, M.-C. Ku, D. M. Jayaseema, J.-S. Lai, D.-Y. Hueng and C. Chang, PLoS One, 2013, 8, e56125 CAS.
- M. Gutova, J. Frank, M. D'Apuzzo, V. Khankaldyyan, M. Gilchrist, A. Annala, M. Metz, Y. Abramyants, K. Herrmann, L. Ghoda, J. Najbauer, C. Brown, M. Blanchard, M. Lesniak, S. Kim, M. Barish, K. Aboody and R. A. Moats, Stem Cells Transl. Med., 2013, 2, 766–775 CrossRef CAS PubMed.
- M. Thu, L. Bryant, T. Coppola, E. Jordan, M. Budde, B. Lewis, A. Chaudhry, J. Ren, N. Varma, A. Arbab and J. Frank, Nat. Med., 2012, 18, 463–467 CrossRef CAS PubMed.
- A. Khurana, H. Nejadnik, F. Chapelin, O. Lenkov, S. Lee, S. N. Gupta, N. Aflakian, N. Derugin, S. Messing, G. Lin, T. F. Lue, L. Pisani and H. E. Daldrup-link, Nanomedicine, 2013, 8, 1969–1983 CrossRef CAS PubMed.
- S. I. Jenkins, M. R. Pickard, N. Granger and D. M. Chari, ACS Nano, 2011, 5, 6527–6538 CrossRef CAS PubMed.
- M. R. Pickard, P. Barraud and D. M. Chari, Biomaterials, 2011, 32, 2274–2284 CrossRef CAS PubMed.
- C. Adams, M. Pickard and D. Chari, Nanomedicine, 2013, 9, 737–741 CrossRef CAS PubMed.
- M. Pickard, S. Jenkins, C. Koller, D. Furness and D. Chari, Tissue Eng., Part C, 2010, 17, 89–99 CrossRef PubMed.
- M. Pickard and D. Chari, Nanomedicine, 2010, 5, 217–232 CrossRef CAS PubMed.
- C. F. Adams, A. Rai, G. Sneddon, H. H. P. Yiu, B. Polyak and D. M. Chari, Nanomedicine, 2015, 11, 19–29 CrossRef CAS PubMed.
- K. Leung, in Molecular Imaging and Contrast Agent Database, 2004 Search PubMed.
- S. Lyer, R. Tietze, S. Dürr, T. Struffert, T. Engelhorn, M. Schwarz, A. Dörfler, L. Budinsky, A. Hess, W. Schmidt, R. Jurgons and C. Alexiou, in Volume 140 of Springer Proceedings in Physics, Springer, 2008, pp. 199–203 Search PubMed.
- T. Gorba and L. Conti, Expert Opin. Drug Delivery, 2013, 8, 1083–1094 CrossRef CAS PubMed.
- A. J. Mothe, I. Kulbatski, A. Parr, M. Mohareb and C. H. Tator, Cell Transplant., 2008, 17, 735–751 Search PubMed.
- S. Casarosa, Y. Bozzi and L. Conti, Mol. Cell. Ther., 2014, 2, 31 CrossRef PubMed.
- G. Maiorano, S. Sabella, B. Sorce, V. Brunetti, M. A. Malvindi, R. Cingolani and P. P. Pompa, ACS Nano, 2010, 4, 7481–7491 CrossRef CAS PubMed.
- G. T. Grottone, R. R. Loureiro, J. Covre, E. B. Rodrigues and J. Á. P. Gomes, Curr. Eye Res., 2013, 1–8 Search PubMed.
- P. Hughes, D. Marshall, Y. Reid, H. Parkes and C. Gelber, Biotechniques, 2007, 43, 575–586 CrossRef CAS PubMed.
- A. Bez, E. Corsini, D. Curti, M. Biggiogera, A. Colombo, R. F. Nicosia, S. F. Pagano and E. A. Parati, Brain Res., 2003, 993, 18–29 CrossRef CAS PubMed.
- M. R. Pickard, C. F. Adams, P. Barraud and D. M. Chari, J. Funct. Biomater., 2015, 6, 259–279 CrossRef CAS PubMed.
- Y. Sanchez-antequera, O. Mykhaylyk, N. P. Van Til, A. Cengizeroglu, J. H. De Jong, M. W. Huston, M. Anton, I. C. D. Johnston, Z. Pojda, G. Wagemaker and C. Plank, Methods Mol. Biol., 2015, 117, 171–182 Search PubMed.
- M. Song, W. K. Moon, Y. Kim, D. Lim, I. C. Song and B. W. Yoon, Korean J. Radiol., 2007, 8, 365 CrossRef PubMed.
- S. S. Eamegdool, M. W. Weible, B. T. T. Pham, B. S. Hawkett, S. M. Grieve and T. Chan-ling, Biomaterials, 2014, 35, 5549 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |