New insights into structure–activity relationships for propane hydrogenolysis over Ni–Cu bimetallic catalysts
Yunxi Yao* and
D. Wayne Goodman†
Department of Chemistry, Texas A&M University, College Station, TX 77843, USA. E-mail: yunxiyao@gmail.com
First published on 8th May 2015
Abstract
Propane hydrogenolysis has been investigated on Ni–Cu/SiO2 model catalysts under elevated pressure conditions. The surface Ni active sites on Ni–Cu/SiO2 were measured by selective hydrogen adsorption under ultrahigh vacuum (UHV) conditions. The specific activity of propane hydrogenolysis shows a slight increase and then decrease with increasing Cu coverages on Ni–Cu/SiO2 bimetallic catalysts, and varies within the 0.6 s−1 to 2.0 s−1 range. On the contrary, many previous studies show a three-to-five order decrease in the specific activity of ethane or propane hydrogenolysis over Ni–Cu bimetallic catalysts. The significant difference was explained by the possible over counting of active Ni sites in the selective hydrogen chemisorptions under atmospheric pressure conditions due to hydrogen spillover from Ni to Cu. New structure–activity relationships for Ni–Cu bimetallic catalysts in propane hydrogenolysis were established based on the present work. Furthermore, possible carbon deposits in this reaction were examined by post reaction Auger electron spectroscopy.
1 Introduction
In the past 40 years, great research interest has been devoted to bimetallic catalysts, which usually show chemical and catalytic properties different from the monometallic counterparts.1–10 The distinct properties of bimetallic catalysts from pure metals are generally explained by ensemble effects and ligand or electronic effects.1–4 In most cases both effects play a role in the surface adsorption and catalytic reactions on bimetallic catalysts.11 There is one typical bimetallic system composed of a Group VIII metal and a Group IB metal, such as Ni–Cu,12–16 Ni–Au,17–20 Pd–Ag,21 Pd–Au,3,22 Ru–Cu,15,23,24 where the active Group VIII metal is diluted by the less active Group IB metal. Ensemble effects usually are considered to play a more significant role in such systems.21,22 Our focus in this paper is to study the surface structure–activity relationships of Ni–Cu bimetallic catalysts.Ni–Cu bimetallic catalysts have become a classic system since the pioneering work of Sinfelt and co-workers on Ni–Cu bimetallic catalysts in 1972 when they found there was a four-to-five order decrease in the specific activity of the Ni–Cu catalysts for ethane hydrogenolysis reactions from pure Ni to Ni–Cu bimetallic catalysts with 60 atomic% Cu.13 Similar phenomena were also observed on Ru–Cu bimetallic catalysts.14 Sinfelt proposed an ensemble effect to explain the sharp decrease of the specific activity of ethane hydrogenolysis on Ni catalysts caused by adding of Cu.1,15 Sinfelt suggested active multiplets are needed for ethane hydrogenolysis on Ni–Cu bimetallic catalysts, and the concentration of such an ensemble will decline sharply because the active Ni metal atoms are diluted with inactive Cu metal atoms.1,15 Dalmon and Martin had proposed that that about 12-to-18 adjacent nickel atoms are required for ethane, propane and butane hydrogenolysis on Ni–Cu/SiO2 catalysts.25 Alstrup and coworkers found the sulfur modification effect on nickel catalysts in propane hydrogenolysis cannot be explained by the advanced ensemble model, which was proposed to interpret the hydrogenolysis results over Ni–Cu bimetallic catalysts by Sinfelt and others,1,15,25 and they suggested breaking of the C–C bonds may need special sites.26
These conclusions are entirely based on the specific activity of the Ni–Cu catalysts for hydrogenolysis reactions, which is usually obtained with the active Ni sites measured by selective hydrogen adsorption assuming no hydrogen uptake on Cu sites on Ni–Cu bimetallic catalysts.1,15,25,26 It is true there is no hydrogen uptake on pure Cu at room temperature due to the activated dissociative hydrogen adsorption on Cu.27 But Cu sites could be possibly occupied by atomic hydrogen by hydrogen spillover from Ni to Cu on bimetallic catalysts, as Crucq et al. observed hydrogen spillover from Ni to Cu on Ni–Cu alloys in their hydrogen adsorption isotherms.28 Recently we got direct evidence that hydrogen spillover from oversaturated Ni sites to Cu sites.12 As suggested by Goodman and coworkers the possible hydrogen spillover on bimetallic catalysts could results in over counting active sites and failure in the hydrogen selective chemisorptions on bimetallic catalysts.12,29 However, our previous study shows the selective hydrogen adsorption on Ni sites is still valid under ultrahigh vacuum (UHV) conditions.12
Another issue in Ni–Cu bimetallic catalysts is that the Cu sites are traditionally considered just as a diluting agent due to its lower activity compared with Ni.13–15 Although pure copper is not active for rupture of C–C, or H–H bonds, the Cu sites surrounding active metals could act as receivers for activated hydrogen or cleaved carbon–hydrogen species by the surrounded active metal sites.30–32
Inspired by these new research findings, we herein re-investigated the surface structure–activity relationships of Ni–Cu bimetallic catalysts for propane hydrogenolysis reactions on a Ni–Cu model catalyst under technique relevant reaction conditions. The total reaction rate of propane hydrogenolysis over Ni–Cu bimetallic catalysts sharply decreased with the increase of Cu coverage. However, the specific surface activity of propane hydrogenolysis (number of propane molecules reacted per Ni active site per second) changed within only one-order, unlike the reported results that a three-to-five order decrease in the specific activity of ethane or propane hydrogenolysis over Ni–Cu bimetallic catalysts. The significant differences between our studies and the literature results are discussed, and new insights of the structure–activity relationship of Ni–Cu bimetallic catalysts were obtained.
2 Experimental section
All the experiments were carried out in a stainless steel ultrahigh vacuum (UHV) chamber with a coupled high pressure cell for adsorption and reactions at elevated pressures.12,33–35 Briefly, the system was equipped with Auger electron spectroscopy (AES), low energy electron diffraction (LEED) and a UTI 100 mass spectrometer for temperature programmed desorption (TPD) measurement. The bottom of the UHV chamber was coupled with a high pressure cell which could be isolated from the main chamber by differentially pumped sliding seals for high pressure studies. The base pressures for both the main chamber and the high pressure cell are lower than 2.0 × 10−10 Torr. A linear ramp of 10 K s−1 was used for H2 TPD measurements.The preparation of Ni–Cu bimetallic catalysts supported on SiO2/Mo(110) was described previously in details.12,33,35 Briefly, Ni and Cu were evaporated on thin SiO2 films supported on Mo(110) substrates via resistive heating of a tantalum filament wrapped with respective high pure metal wires. The supported Ni–Cu bimetallic catalysts were annealed at 700 K for 5 min before any measurements. For all the measurements, the rear sides of Mo(110) substrates were covered with SiO2 thin films without metal depositions. The amount of metal deposited was controlled by depositing time, calibrated with AES spectra, and was given in the unit of monolayer equivalent (MLE, 1 MLE = 1.43 × 1015 atom per cm2).12,35 Ni–Cu will grow into nanoparticles on SiO2 thin films. It has been measured the average particle size is around 4 nm for 5 MLE Ni/SiO2.33
Ultrahigh purity H2 was further purified by passing through a LN2 cooling trap. Propane was purified by repeated freeze–pump–thaw cycles before mixed with hydrogen. The propane hydrogenolysis reactions were carried out in the high pressure cell as a batch reactor (1.2 L) using C3H8/H2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) mixtures at a total pressure of 60 Torr at 600 K for 20 min. The conversions of propane were controlled below 5%. The reaction products were analyzed with a HP 5890 gas chromatograph equipped with a flame ionization detector and a Porak Q column. Blank experiments on SiO2 films showed that the rate of propane hydrogenolysis was four orders lower than monometallic Ni/SO2 under the same reaction conditions.
5) mixtures at a total pressure of 60 Torr at 600 K for 20 min. The conversions of propane were controlled below 5%. The reaction products were analyzed with a HP 5890 gas chromatograph equipped with a flame ionization detector and a Porak Q column. Blank experiments on SiO2 films showed that the rate of propane hydrogenolysis was four orders lower than monometallic Ni/SO2 under the same reaction conditions.
3 Results and discussion
3.1 Reaction rates of propane hydrogenolysis over Ni–Cu/SiO2
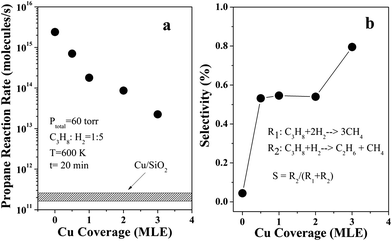
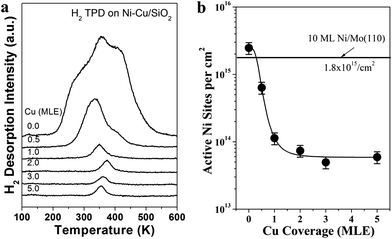
Fig. 1(a) shows the reaction rates of propane hydrogenolysis at 600 K over Ni–Cu/SiO2 bimetallic catalysts as a function of Cu coverage on Cu-5 MLE Ni/SiO2. The reaction rates of propane hydrogenolysis sharply decrease by two orders in magnitude with increasing Cu coverages from 0 to 3 MLE. The reaction rate of propane hydrogenolysis on pure Cu (5 MLE Cu/SiO2) was also measured, which is about four orders lower than that of the monometallic Ni (5 MLE Ni/SiO2), within the same order as background reaction on clean SiO2 thin films. These observations agree well with previous reported results that the reaction rate of ethane hydrogenolysis decreased markedly with addition of Cu to Ni catalysts.13–15 There are two possible reactions for propane hydrogenolysis: (i) rupture of both the C–C bonds in the propane to form methane; (ii) rupture of only one C–C bond in the propane to form methane and ethane. The selectivity of reaction (ii) was shown in Fig. 1(b), which is consistent with reported results that the selectivity for partial hydrogenolysis increases with increasing Cu coverage.25 On clean Ni surface, the selectivity is only 5%, which indicates almost all the C–C bonds are fully ruptured due to the strong interaction between propane and Ni surfaces. The increased selectivity can be explained by ensemble effect. The decreased Ni–Ni adjacent site number by adding of Cu will also account for the decreased possibility for propane complete hydrogenolysis.It has been shown that the active Ni sites can be measured by selective H2 adsorption under UHV conditions without the possible hydrogen spillover effect from Ni to Cu.12 H2 TPD on Ni–Cu/SiO2 as a function of Cu coverage, as shown in Fig. 2(a), indicates all hydrogen desorbs from Ni sites without H2 desorption features on Cu sites, which will appear at lower desorption temperatures.12 One interesting feature from Fig. 2(a) is that the H2 desorption peaks first increase to 372 K with increasing Cu coverage from 0 to 2 MLE, and then slowly decrease to 354 K at 5 MLE Cu coverage. The first increase of the hydrogen desorption temperature may be caused by the decrease of molecule–molecule interaction by adding Cu on the surface. When Ni sites are highly isolated by Cu atoms at high Cu coverages, the adsorption of hydrogen on Ni sites can be weakened due to the electronic modification effect by Cu to Ni. The surface Ni active sites could be estimated by comparing the H2 TPD desorption intensity on Ni–Cu/SiO2 with H2 TPD desorption intensity on a 10 ML Ni/Mo(110) film, as shown in Fig. 2(b). Since multilayer Ni films on a Mo(110) mainly expose (111) facet, it is reasonable to assume the surface atomic density of the 10 ML Ni/Mo(110) equal to that of the Ni(111) surface (1.8 × 1015 atoms per cm2).33 Combined the H2 TPD results with the known surface area of the Mo(110) (0.78 cm2), the active sites could be estimated. The H2 TPD intensity on 1 MLE Cu-5 MLE Ni/SiO2 is just about 5% of that on 5 MLE Ni/SiO2, which indicates Cu enriches on the Ni–Cu alloy surfaces due to the lower surface free energy of Cu than Ni.35–38 After that, the H2 desorption intensity remains almost constant independent of the Cu coverage. The trend of the evolution of H2 TPD intensity with Cu coverage looks similar to the reaction rate of propane hydrogenolysis as reported in Fig. 1(a).
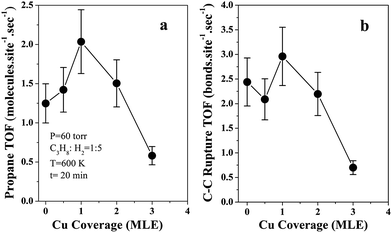
In order to get more clear surface structure–activity relationship, specific surface activity of propane turn over frequency (TOF, number of propane molecules consumed per active site per second) as a function of Cu coverage for propane hydrogenolysis reaction over Ni–Cu/SiO2 was plotted in Fig. 3(a) by using the measured reaction rates of propane hydrogenolysis (Fig. 1) and the estimated Ni active sites (Fig. 2). The propane TOF slightly increases from 1.2 s−1 on clean 5 MLE Ni/SiO2 to 2.0 s−1 at 1 MLE Cu coverage, and then decreases to 0.6 s−1 at 3 MLE Cu coverage. The first increase in the propane TOF is mainly because the increase of selectivity of ethane products with adding of Cu on Ni. If the specific reaction rate is plotted as the turn over frequency of C–C bond ruptures, as shown in Fig. 3(b), the turn over frequency of C–C rupture changes only from 2.3 s−1 on pure 5 MLE Ni/SiO2 to 2.9 s−1 on 1 MLE Cu–Ni/SiO2. The specific activity of Ni toward C–C rupture does not change with Cu coverages up to 1 MLE. But the complete hydrogenolysis of propane to methane is hindered by adding of Cu, which results in the slight increase in the specific activity of propane hydrogenolysis. With Cu coverages above 1 MLE, the continuous decrease of propane TOF may be caused by the decreased activity of Ni when surrounded and isolated by Cu atoms at high Cu coverages. The electronic effect leads to the decrease of the specific activity.39 It has been reported that the adsorption energy of CHx decreases on Ni when alloyed with Cu.40 Our results show the specific activity of Ni sites for propane hydrogenolysis could be tuned by adding of Cu, but varies just in a small relative range (0.4 to 2.0 s−1). It has been reported that there was a three-to-five order decrease in the specific reaction rate of ethane or propane hydrogenolysis over Ni–Cu bimetallic catalysts with 50 atomic% Cu compared with that on pure Ni catalysts.13–15,25,26 Our data presented here are significantly different from those reported results. The uncertainties in the Ni active site measurement from H2 TPD are estimated less than 20%,41 which will not change the trend observed in the propane hydrogenolysis specific activity.
The difference is probably caused by the over counting of Ni sites in the active site measurement by selective hydrogen chemisorptions usually conducted at atmospheric pressure conditions for technique bimetallic catalysts characterizations,7 where hydrogen spillover from Ni to Cu is kinetically possible.12 Our recent reports show direct evidence that saturated atomic hydrogen on Ni could spillover to Cu on Ni–Cu bimetallic catalysts.12 Crucq et al. observed hydrogen spillover from Ni to Cu on Ni–Cu alloys in their hydrogen adsorption isotherms.28 On Ni–Cu bimetallic catalysts, Cu is enriched on the surface due to its lower surface free energy.35 From clean monometallic Ni catalysts to Ni–Cu bimetallic catalysts with high Cu concentrations, several order decrease in the surface Ni sites can be expected, which is the main reason for sharp decrease in the total reaction rate in hydrogenolysis. The correctness in the measurement of the specific reaction rate highly depends on the reliability of the surface active site measurements. Any adsorbate spillover or surface segregation induced by adsorption can lead to large discrepancy in the counting of surface active sites via selective chemisorptions.12,35 Goodman et al. had reported that hydrogen spillover from Ru to Cu happens on Ru–Cu bimetallic catalysts, resulting in the active site over counted by selective hydrogen chemisorptions.23,24,29 Yao and Goodman have shown CO adsorption is not suitable for Ni surface site measurement on Ni–Cu bimetallic catalysts even under UHV conditions due to CO adsorption induced Ni surface segregation.
Moreover, our results here indicate the proposal that a large ensemble of Ni atoms is required for hydrogenolysis reactions over Ni–Cu bimetallic catalysts may be not true.1,25 Certain atom ensembles, not limited to adjacent Ni sites, may be required to accommodate the C–C rupture in the hydrogenolysis reactions. Cu atoms around Ni sites could play a more important role rather than traditionally considered as diluting inert agents, such as acting as receivers for hydrogen or carbon hydrogen species activated by Ni sites.30,31 Recently Medlin et al. reported synergistic effect was observed in Cu4Ni alloy catalysts in hydrogenation reactions due to a hydrogen spillover effect from Ni to Cu.32 The determination of the smallest atomic ensembles for the C–C rupture in the hydrogenolysis reactions is out of the scope of this paper. But a large ensemble of 12-to-18 adjacent nickel atoms proposed by Dalmon and Martin is not necessary for this reaction based on our present results.25
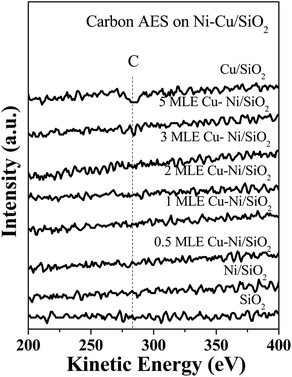
The possible carbon deposits in the propane hydrogenolysis over Ni–Cu/SiO2 were studied by post-reaction AES spectra, as shown in Fig. 4. After annealing to 600 K (reaction temperature) in UHV, there are no carbon deposits detected on Ni–Cu/SiO2 bimetallic catalysts. This results show under the reaction conditions there is no strong adsorbed carbon species deposited on the Ni–Cu surfaces excluding the possible influence of carbon deposits in the hydrogenolysis reactions.25 But there is relative amount carbon deposits detected on the monometallic Cu catalysts of 5 MLE Cu/SiO2, which agrees with that carbon layers could form on Cu surfaces by cracking of carbon hydrogen species.42 This result indicates carbon deposits could take place on Cu rich Ni–Cu surfaces due to the lack of active Ni sites to remove carbon deposits by hydrogenation reactions.
Another effect on the surface reactivity of Ni–Cu/SiO2 bimetallic catalysts is the possible surface segregation of Ni under propane hydrogenolysis reaction conditions. We have shown CO adsorption could induce Ni surface segregation due to strong interaction between CO and Ni.35 Molecular propane has weak interaction with Ni, but the dissociated carbon hydrogen species could have strong interaction with Ni. However, these reaction intermediates will be quickly hydrogenated and have low surface coverages. Our present results do not show obvious Ni surface segregation effect in propane hydrogenolysis reactions, but the possibility cannot be excluded.
4 Conclusions
Propane hydrogenolysis reactions were investigated on Ni–Cu/SiO2 model catalysts. The total reaction rate of propane hydrogenolysis sharply decreases on Ni–Cu bimetallic catalysts as a function of Cu coverage due to the surface enrichment of Cu. The selectivity for ethane formation in propane hydrogenolysis is enhanced by adding of Cu to Ni on Ni–Cu bimetallic catalysts. Specific activity of propane hydrogenolysis is slightly affected by adding of Cu to Ni on Ni–Cu bimetallic catalysts, which varies in the range of 0.6 to 2.0 s−1. The significant differences of the specific activity of propane hydrogenolysis over Ni–Cu bimetallic catalysts between the present study and those reported in literature are mainly because the possible over counting of the active Ni sites in the selective hydrogen chemisorptions, where hydrogen spillover from Ni to Cu could occur under atmospheric pressure conditions. This research gains new insights into the surface structure–activity relationships for Group VIII-Group IB bimetallic catalysts, e.g. Ni–Cu, which will benefit the designs of highly selective catalysts.Acknowledgements
This work was financially supported by the US Department of Energy, Office of Basic Energy Sciences, Division of Chemical Sciences, Geosciences, and Bio-sciences (DE-FG02-95ER-14511).Notes and references
- J. H. Sinfelt, Bimetallic catalysts:discovreries, concepts, and applications, Wiley, New York, 1983 Search PubMed.
- J. A. Rodriguez, Surf. Sci. Rep., 1996, 24, 223 CrossRef CAS.
- F. Gao and D. W. Goodman, Chem. Soc. Rev., 2012, 41, 8009 RSC.
- F. Maroun, F. Ozanam, O. M. Magnussen and R. J. Behm, Science, 2001, 293, 1811 CrossRef CAS PubMed.
- W. Yu, M. D. Porosoff and J. G. Chen, Chem. Rev., 2012, 112, 5780 CrossRef CAS PubMed.
- Z. Wei, J. Sun, A. K. Datye and Y. Wang, Chem. Soc. Rev., 2012, 41, 7994 RSC.
- C. T. Campbell, Annu. Rev. Phys. Chem., 1990, 41, 775 CrossRef CAS.
- J. A. Rodriguez and D. W. Goodman, Science, 1992, 257, 897 CAS.
- J. K. Nørskov, F. Abild-Pedersen, F. Studt and T. Bligaard, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 937 CrossRef PubMed.
- F. Tao, M. E. Grass, Y. Zhang, D. R. Butcher, J. R. Renzas, Z. Liu, J. Y. Chung, B. S. Mun, M. Salmeron and G. A. Somorjai, Science, 2008, 322, 932 CrossRef CAS PubMed.
- Q. Li, L. Song, L. Pan, X. Zhuang, M. Ling and L. Duan, Phys. Chem. Chem. Phys., 2013, 15, 20345 RSC.
- Y. X. Yao and D. W. Goodman, J. Mol. Catal. A: Chem., 2014, 383–384, 239 CrossRef CAS PubMed.
- J. H. Sinfelt, J. L. Carter and D. J. C. Yates, J. Catal., 1972, 24, 283 CrossRef CAS.
- J. H. Sinfelt, J. Catal., 1973, 29, 308 CrossRef CAS.
- J. H. Sinfelt, Acc. Chem. Res., 1977, 10, 15 CrossRef CAS.
- E. Demirci, C. Carbogno, A. Groβ and A. Winkler, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 80, 085421 CrossRef.
- F. Yang, Y. X. Yao, Z. Yan, H. Min and D. W. Goodman, Appl. Surf. Sci., 2013, 283, 263 CrossRef CAS PubMed.
- S. A. Tenney, W. He, C. C. Roberts, J. S. Ratliff, S. I. Shah, G. S. Shafai, V. Turkowski, T. S. Rahman and D. A. Chen, J. Phys. Chem. C, 2011, 115, 11112 CAS.
- Z. J. Wang, Q. Fu, Z. Wang and X. H. Bao, Surf. Sci., 2012, 606, 1313 CrossRef CAS PubMed.
- Z. Yan, Y. X. Yao and D. W. Goodman, Catal. Lett., 2012, 142, 714–717 CrossRef CAS.
- L. A. Mancera, R. J. Behm and A. Groβ, Phys. Chem. Chem. Phys., 2013, 15, 1497 RSC.
- M. Chen, D. Kumar, C. W. Yi and D. W. Goodman, Science, 2005, 310, 291 CrossRef CAS PubMed.
- C. H. F. Peden and D. W. Goodman, Ind. Eng. Chem. Fundam., 1986, 25, 58 CAS.
- D. W. Goodman, J. T. Yates Jr and C. H. F. Peden, Surf. Sci., 1985, 164, 417 CrossRef CAS.
- J. A. Dalmon and G. A. Martin, J. Catal., 1980, 66, 214 CrossRef CAS.
- I. Alstrup, U. E. Petersen and J. R. Rostrup-Nielsen, J. Catal., 2000, 191, 401 CrossRef CAS.
- F. Greuter and E. W. Plummer, Solid State Commun., 1983, 48, 37 CrossRef CAS.
- A. Crucq, L. Degols, G. Lienard and A. Frennet, Stud. Surf. Sci. Catal., 1983, 17, 137 CrossRef.
- D. W. Goodman and C. H. F. Peden, J. Catal., 1985, 95, 321 CrossRef CAS.
- M. D. Marcinkowski, A. D. Jewell, M. Stamatakis, M. B. Boucher, E. A. Lewis, C. J. Murphy, G. kyriakou and E. C. H. Sykes, Nat. Mater., 2013, 12, 523 CrossRef CAS PubMed.
- G. Kyriakou, M. B. Boucher, A. D. Jewell, E. Lewis, T. Lawton, A. E. Baber, H. L. Tierney, M. Flytzani-Stephanopoulos and E. C. H. Sykes, Science, 2012, 335, 1209 CrossRef CAS PubMed.
- S. H. Pang, N. E. Love and J. W. Medlin, J. Phys. Chem. Lett., 2014, 5, 4110 CrossRef CAS.
- Y. X. Yao, Z. Yan, L. Chen, Z. Zhou and D. W. Goodman, Catal. Lett., 2012, 142, 1437 CrossRef CAS.
- Y. X. Yao, Q. Fu, Y. Y. Zhang, X. Weng, H. Li, M. S. Chen, L. Jin, A. Dong, R. Mu, P. Jiang, L. Liu, H. Bluhm, Z. Liu, S. B. Zhang and X. H. Bao, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 17023 CrossRef CAS PubMed.
- Y. X. Yao and D. W. Goodman, Phys. Chem. Chem. Phys., 2014, 16, 3823 RSC.
- H. H. Brongersma and M. J. Sparnany, Surf. Sci., 1978, 71, 657 CrossRef CAS.
- S. M. Foiles, Phys. Rev. B: Condens. Matter Mater. Phys., 1985, 32, 7685 CrossRef CAS.
- A. R. Naghash, T. H. Etsell and S. Xu, Chem. Mater., 2006, 18, 2480 CrossRef CAS.
- C. O. Bennett and M. Che, J. Catal., 1989, 120, 293 CrossRef CAS.
- H. Liu, R. Zhang, R. Yan, J. Li, B. Wang and K. Xie, Appl. Surf. Sci., 2012, 258, 8177 CrossRef CAS PubMed.
- S. M. McClure, M. Lundwall, F. Yang, Z. Zhou and D. W. Goodman, J. Phys. Chem. C, 2009, 113, 9688 CAS.
- X. Li, W. Cai, J. An, S. Kim, J. Nah, D. Yang, R. Piner, A. Velamakanni, I. Jung, E. Tutuc, S. K. Banerjee, L. Colombo and R. S. Ruoff, Science, 2009, 324, 1312 CrossRef CAS PubMed.
Footnote |
| † Dr Goodman deceased on February 27, 2012. |
| This journal is © The Royal Society of Chemistry 2015 |