A multifunctional molecular entity CuII–SnIV heterobimetallic complex as a potential cancer chemotherapeutic agent: DNA binding/cleavage, SOD mimetic, topoisomerase Iα inhibitory and in vitro cytotoxic activities†
Sartaj Tabassum*a,
Ahmad Asima,
Rais Ahmad Khanb,
Farukh Arjmanda,
Dhivya Rajakumarc,
Perumalsamy Balajic and
Mohammad Abdulkader Akbarshade
aDepartment of Chemistry, Aligarh Muslim University, Aligarh-202002, India. E-mail: tsartaj62@yahoo.com
bDepartment of Chemistry, King Saud University, Riyadh, 11451, Kingdom of Saudi Arabia
cDepartment of Biomedical Science, Bharathidasan University, Tiruchirappalli 620 024, India
dMahatma Gandhi-Doerenkamp Center (MGDC) for Alternatives to Use of Animals in Life Science Education, Bharathidasan University, Tiruchirappalli 620 024, India
eDepartment of Food Science and Nutrition, College of Food Science and Agriculture, Jing Saud University, Riyadh, Kingdom of Saudi Arabia
First published on 5th May 2015
Abstract
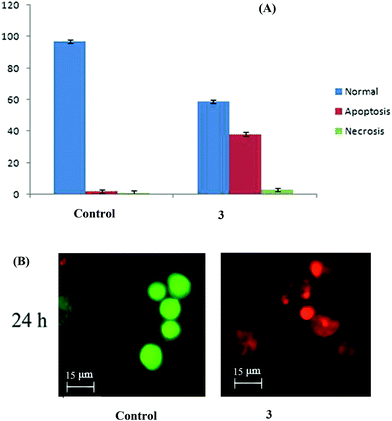
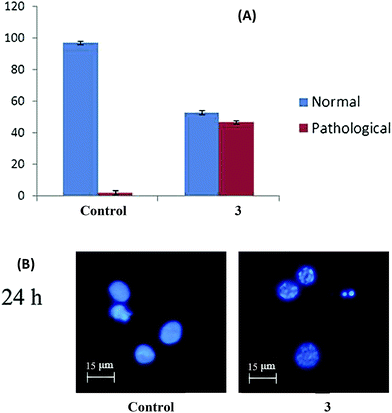
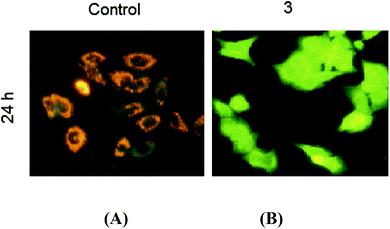
New chiral L-valine-derived Schiff base complexes with the bioactive heterocyclic ligand scaffold pyrazole (Hpz) were designed and synthesized with a view to find their potential as anticancer chemotherapeutic drug candidates. The monometallic chemical entities CuII (1) and ZnII (2), and heterobimetallic CuII–SnIV (3), and ZnII–SnIV (4) were synthesized and characterized adopting various spectroscopic (UV-vis, IR, 1H 13C and 119Sn NMR, EPR and ESI-MS) and analytical methods. In vitro, CT-DNA-binding profiles of 1–4 were studied by UV-vis, fluorescence spectroscopy, and circular dichroism. The results display the significantly higher binding affinity of heterobimetallic complexes 3 and 4 than monometallic complexes 1 and 2. This could be attributed to the dual binding mode facilitating a preferential electrostatic interaction via SnIV towards the surface phosphate oxygen of the sugar–phosphate backbone of the DNA double helix, in addition to the covalent overlap of CuII to N7 of the guanine of the DNA. Moreover, complex 3 exhibited significantly efficient DNA cleavage activity involving the formation of the superoxide radical as well as the hydroxyl radical as the reactive species. The SOD-like activity of 3 and 4 was evaluated using a xanthine/xanthine oxidase assay, which showed SOD activity in the micromolar range for both the heterobimetallic complexes viz., (IC50) 0.082 μM for 3 and 12 μM for 4. Furthermore, 3 showed high inhibitory activity against Topo-Iα at a concentration of 20 μM as IC50, suggesting that complex 3 is an efficient DNA cleaving agent. In vitro studies on the anticancer activity against the HepG2 hepatocellular carcinoma cell line revealed that both complexes 3 and 4 have the capability to kill the chosen cancer cell, but the efficiency of complex 3 is 10 times higher than 4. The mode of cell death induced by complex 3 is primarily apoptosis as revealed by AO/EB staining, Hoechst 33258 staining, and assessment of the mitochondrial trans-membrane potential.
Introduction
Cancer is a deadly menace, involving unregulated cell growth, invasion, and metastasis.1 Cancer is the third leading cause of death with ∼12 million new cases, and an estimated 7.6 million cancer deaths to have occurred globally in 2007.2 By 2030, this figure is expected to have increased exponentially (increase by +69%).3 The current treatment modalities include: (i) surgical procedures, which provide the most successful cure, followed by (ii) several chemotherapy regimens, which substantially improve local relapse rates and the overall survival of the patient, (iii) immunotherapy or hormone therapy,4a and (iv) radiotherapy.4b Neoadjuvant chemotherapy is administered earlier to the main treatment to reduce the size or extent of cancer before using a radical treatment intervention, thus making procedures easier and more likely to be successful.5 Globally, the sixth most frequent cancer is primary liver cancer. It is ranked as the second leading cause of cancer death. Only in 2012, the cases found were 782![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and led to 746
000 and led to 746![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 deaths.6 Thus, liver cancer is a principal health concern. The chemotherapeutic drugs employed currently suffer from the development of resistance or are not specific to the target so that systemic toxicities arise.7 Transarterial chemoembolization of doxorubicin using drug-eluting beads is recommended as the principal intervention for liver cancer. Metal-based chemical entities are also in the pipeline in light of cisplatin having stimulated interest and spurred research into new metal-based anticancer drugs. Copper is an essential trace metal and regulates angiogenesis, i.e., the growth of tumor blood vessels that are important for tumor growth, invasion, and metastasis.8 It has been shown that tumors without adequate blood supply do not grow larger than 1 to 2 mm.3,9 A potential strategy for cancer chemotherapy is an organic scaffold with metallic species, particularly copper, that can bind with elevated copper in cancer cells and tissues. The accumulation of a high concentration of copper is a unique feature of cancer cells, which while leaving normal cells to suffer only minimally thereby converts pro-angiogenic cofactor copper into a cancer-specific killing agent.10 On the other hand, organotin(IV) is considered as an important class of compounds for fighting cancer. A large number of compounds have shown efficient cytotoxic activities against panels of human cancer cell lines both in vitro and in vivo.11 Among the organic ligands, Schiff bases are the most appropriate ligands owing to their versatile synthesis, metal binding domain, and novel structural features.12 A Schiff base can prove to be an ideal functional pharmacophore for metal-based drug design. Such Schiff base complexes are seldom reported. In the case of new Schiff base complexes, it is important to evaluate the mode of binding with DNA, the extent of binding and the cleavage mechanism13 that are the pre-criteria for efficacious metallodrugs for cancer. The Schiff base of O-vanillin and L-valine has unique structural features due to the presence of biocompatible motif viz., an amino acid, in combination with a biologically active pyrazole ancillary ligand.
000 deaths.6 Thus, liver cancer is a principal health concern. The chemotherapeutic drugs employed currently suffer from the development of resistance or are not specific to the target so that systemic toxicities arise.7 Transarterial chemoembolization of doxorubicin using drug-eluting beads is recommended as the principal intervention for liver cancer. Metal-based chemical entities are also in the pipeline in light of cisplatin having stimulated interest and spurred research into new metal-based anticancer drugs. Copper is an essential trace metal and regulates angiogenesis, i.e., the growth of tumor blood vessels that are important for tumor growth, invasion, and metastasis.8 It has been shown that tumors without adequate blood supply do not grow larger than 1 to 2 mm.3,9 A potential strategy for cancer chemotherapy is an organic scaffold with metallic species, particularly copper, that can bind with elevated copper in cancer cells and tissues. The accumulation of a high concentration of copper is a unique feature of cancer cells, which while leaving normal cells to suffer only minimally thereby converts pro-angiogenic cofactor copper into a cancer-specific killing agent.10 On the other hand, organotin(IV) is considered as an important class of compounds for fighting cancer. A large number of compounds have shown efficient cytotoxic activities against panels of human cancer cell lines both in vitro and in vivo.11 Among the organic ligands, Schiff bases are the most appropriate ligands owing to their versatile synthesis, metal binding domain, and novel structural features.12 A Schiff base can prove to be an ideal functional pharmacophore for metal-based drug design. Such Schiff base complexes are seldom reported. In the case of new Schiff base complexes, it is important to evaluate the mode of binding with DNA, the extent of binding and the cleavage mechanism13 that are the pre-criteria for efficacious metallodrugs for cancer. The Schiff base of O-vanillin and L-valine has unique structural features due to the presence of biocompatible motif viz., an amino acid, in combination with a biologically active pyrazole ancillary ligand.
We describe the syntheses of new monometallic and heterobimetallic complexes with a chiral Schiff base motif (derived from O-vanillin and L-valine) and pyrazole as a secondary ligand. In vitro DNA binding and cleavage, studies were carried out to investigate the potential of the complexes as anticancer drug candidates. The cytotoxicity of the heterobimetallic complexes was investigated in a HepG2 (hepatocellular carcinoma) cell adopting MTT assay and a few assays for apoptosis to find their potential as candidates for the drug development of cancer drugs in general and liver cancer in particular wherein complex 3 was found to be the most efficient.
Results and discussion
Synthesis and characterization
Bioactive chiral complexes of the L-valine Schiff base scaffold with pyrazole in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio of the formulation [Cu(Van–val)(HPz)2] (1), [Zn(Van–val)(Hpz)2] (2); and heterobimetallic entities [Cu(Van–val)(pz)2Sn(CH3)2(H2O)2] (3) and [Zn(Van–val)(pz)2Sn(CH3)2(H2O)2] (4), which were achieved by further addition of (CH3)2SnCl2 to complexes 1 and 2 (Scheme 1). The spectroscopic studies of 1–4 revealed the penta-coordinate coordination geometry of the central metal ions viz., CuII and ZnII while the SnIV atom exhibited an octahedral environment. All these complexes are soluble in DMSO. The molar conductance values of all the complexes 1–4 in DMSO (1 × 10−3 M) at 25 °C were observed in the range 7.4–8.8 ohm−1 cm2 mol−1, which ascertained the non-electrolytic nature of the complexes.
2 molar ratio of the formulation [Cu(Van–val)(HPz)2] (1), [Zn(Van–val)(Hpz)2] (2); and heterobimetallic entities [Cu(Van–val)(pz)2Sn(CH3)2(H2O)2] (3) and [Zn(Van–val)(pz)2Sn(CH3)2(H2O)2] (4), which were achieved by further addition of (CH3)2SnCl2 to complexes 1 and 2 (Scheme 1). The spectroscopic studies of 1–4 revealed the penta-coordinate coordination geometry of the central metal ions viz., CuII and ZnII while the SnIV atom exhibited an octahedral environment. All these complexes are soluble in DMSO. The molar conductance values of all the complexes 1–4 in DMSO (1 × 10−3 M) at 25 °C were observed in the range 7.4–8.8 ohm−1 cm2 mol−1, which ascertained the non-electrolytic nature of the complexes.
The IR spectra of all four complexes 1–4 possess strong characteristic bands attributed to the stretching vibration of azomethine [ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)] in the range 1653–1628 cm−1. A distinctive sharp band associated with ν(C
N)] in the range 1653–1628 cm−1. A distinctive sharp band associated with ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) of the coordinating pyrazole ligands appears at ca. 1569–1551 cm−1 (ref. 14) in complexes 1–4. The bands in the range 1358–1305 cm−1 correspond to the symmetric (COO) stretching vibrations.15 The significant shifts in these values confirm the coordination of the metal center with the tridentate Schiff base ligand (Van–valH). The presence of medium intensity bands at 595–550, 440–425 cm−1 corresponding to ν(M–O), and ν(M–N) vibrations, respectively, ascertain the formation of a coordinate bond with the metal center in all the complexes.16 Furthermore, the IR spectra of complexes 3 and 4 give rise to new peaks associated with ν(Sn–O) and ν(Sn–N) vibrations ∼556–554 cm−1 and 486–482 cm−1, respectively, which further confirms the proposed structure.17
N) of the coordinating pyrazole ligands appears at ca. 1569–1551 cm−1 (ref. 14) in complexes 1–4. The bands in the range 1358–1305 cm−1 correspond to the symmetric (COO) stretching vibrations.15 The significant shifts in these values confirm the coordination of the metal center with the tridentate Schiff base ligand (Van–valH). The presence of medium intensity bands at 595–550, 440–425 cm−1 corresponding to ν(M–O), and ν(M–N) vibrations, respectively, ascertain the formation of a coordinate bond with the metal center in all the complexes.16 Furthermore, the IR spectra of complexes 3 and 4 give rise to new peaks associated with ν(Sn–O) and ν(Sn–N) vibrations ∼556–554 cm−1 and 486–482 cm−1, respectively, which further confirms the proposed structure.17
The 1H NMR spectrum of complex 2 exhibited a characteristic broad peak at 7.57 ppm associated with the NH of pyrazole, whereas the NH signal disappeared from the spectrum of 4. The significant shifts in the signal of the pyrazole protons of 4 (7.53–7.51, 7.16–7.11 ppm) in comparison with 2 (7.77–7.76, 7.14–7.12 ppm), confirms the coordination of SnIV to the azole nitrogen atom via deprotonation. Along with these marked differences, the signals associated with the aromatic and aliphatic protons were observed in accordance with the proposed structure. The multiplicity pattern and the 2J[119Sn,1H] coupling constant values of the dimethyl tin moiety were calculated by the 1H NMR chemical shift. The dihedral C–Sn–C angle was evaluated using Lockhart’s equation. The coupling constant (2J[1H,119Sn]) value was found to be ∼93 and the dihedral C–Sn–C angle obtained was ∼151.2° for complex 4, which is consistent with a hexacoordinated environment around the SnIV atom.18
The 13C NMR spectra showed characteristic signals associated with O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, H–C
O, H–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, and Hpz moiety carbons at 169.2, 168.5, 138.4–127.3 ppm for 2 and with significant downfield shift for 4 at 176.2, 175.8, 136.0–126.9 ppm, respectively. These downfield shifts in signals ascertain the coordination of the Sn(IV) atom with the nitrogen of the pyrazole moieties. Furthermore, phenyl ring aromatic carbon signals were observed in the range 118.2–99.5 ppm for complex 2 and 118.3–103.3 ppm for 4. The peaks associated with the methyl groups bonded to the Sn(IV) atom were found at 18.9 and 17.7 ppm. The coupling constant 1J(13C,119Sn) value for 4 was ∼941 Hz.
N, and Hpz moiety carbons at 169.2, 168.5, 138.4–127.3 ppm for 2 and with significant downfield shift for 4 at 176.2, 175.8, 136.0–126.9 ppm, respectively. These downfield shifts in signals ascertain the coordination of the Sn(IV) atom with the nitrogen of the pyrazole moieties. Furthermore, phenyl ring aromatic carbon signals were observed in the range 118.2–99.5 ppm for complex 2 and 118.3–103.3 ppm for 4. The peaks associated with the methyl groups bonded to the Sn(IV) atom were found at 18.9 and 17.7 ppm. The coupling constant 1J(13C,119Sn) value for 4 was ∼941 Hz.
The 119Sn NMR spectrum of 4 displayed signals at −580.8 ppm. The 119Sn NMR chemical shift values validate the number and type of substituents linked to the tin atom. By applying the Lockhart and Holecek equations, the coupling constants 2J[1H,119Sn] and 1J[13C,119Sn] were used to reveal the stereochemistry of the tin atom, which permitted the calculation of the magnitude of the C–Sn–C bond angles. The chemical shift value is consistent with the hexacoordinated geometry of the tin atom and with those reported earlier.19
The EPR spectra of 1 and 3 in DMSO at LNT were recorded in the X-band region (ESI, Fig. S1†). The g factors were quoted relative to the standard marker TCNE (g = 2.00277). The EPR spectra of complexes 1 and 3 exhibited g‖ = 2.146, 2.213 and g⊥ = 2.057, 2.065 values, respectively. The frozen solution EPR spectra of complexes 1 and 3 revealed axial features (g‖ > g⊥ > 2.0023) and suggested a dx2−y2 ground state. The measurement of the exchange interaction between the copper centers in the polycrystalline compound can be given by the geometric parameter “G” using the relation: G = (g‖ − 2.0023)/(g⊥ − 2.0023). The exchange interaction may be negligible if G is greater than 4. A considerable exchange interaction is indicated in the solid complex if G is less than 4.20 The value g‖ > g⊥ > 2.0023 indicates a dx2−y2 or dz2 ground state that is consistent with square pyramidal geometry. The magnetic moments observed for complexes 1 and 3 are 1.79 BM and 1.85 BM, which are consistent with values expected for copper(II) with the spin S = 1/2.
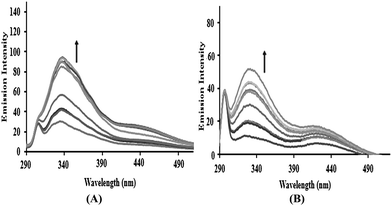
In vitro DNA binding studies
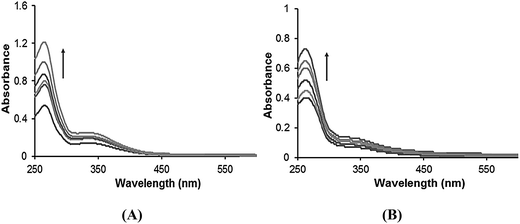 | ||
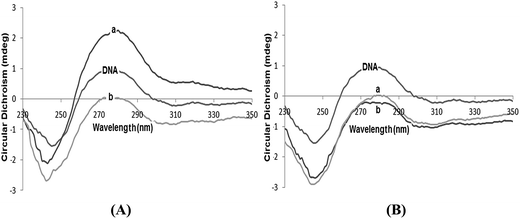
| Fig. 1 The absorption spectral traces of (A) complex 3 and (B) complex 4 in 5% DMSO/5 mM Tris–HCl/50 mM NaCl buffer (pH 7.2), upon increasing the concentration of CT-DNA. | ||
The binding strength of complexes 1–4 with CT-DNA was quantitatively compared by evaluating the intrinsic binding constants Kb of the complexes, which were determined using eqn (1) and by monitoring the changes in absorbance of the π–π* bands with increasing concentrations of CT-DNA:
| [DNA]/|εa − εf| = [DNA]/|εb − εf| + 1/Kb|εb − εf| | (1) |
| Io/I = 1 + KSVr |
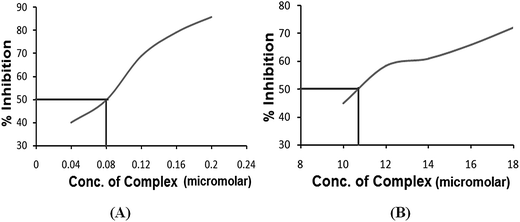 | ||
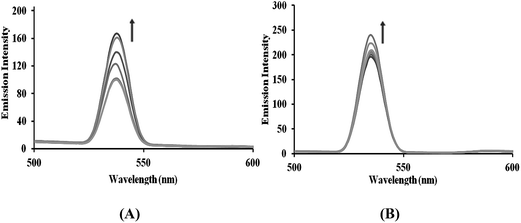
| Fig. 6 The plots of the percentage of NBT inhibition reduction with an increase in the concentration of (A) complex 3 and (B) complex 4. | ||
In addition, to ascertain the effectiveness of the present complexes as functional SOD mimics, we compared the IC50 of the complexes with several standard drugs that were previously determined using the NBT assay (Table 1).29,30
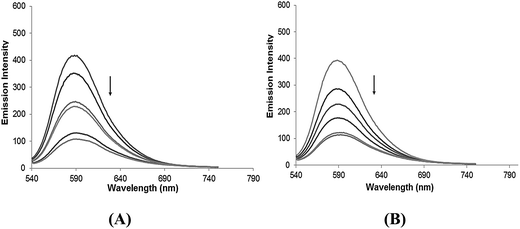
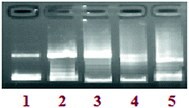
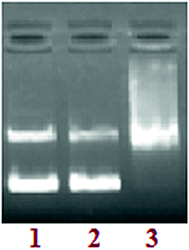
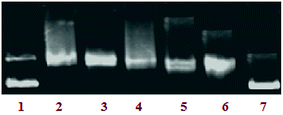
DNA cleavage studies
In vitro anticancer activity
| Synthetic complex/standard drug | IC50 for 24 h (μM) |
|---|---|
| 3 | 6.2 ± 0.10 |
| 4 | 70.0 ± 0.10 |
| Cisplatin | 7.2 ± 0.04 |
Conclusion
The results pertaining to the activity of complex 3 [Cu(Van–val)(pz)2Sn(CH3)2(H2O)2] reveals it to be an efficient DNA-cleaving agent through the dual binding mode. Based on the evidence set forth by DNA-damaging agents as potential anticancer agents, the exploration of complex 3 for antitumor activity on human hepatocarcinoma (HepG2) cells revealed that complex 3 induces cell death greatly through apoptosis, and to a certain extent through ROS-mediated necrosis, and effectively when compared to cisplatin. Further exploration of complex 3, including with a few more tumor cell lines and a normal cell, to understand the selectivity and the molecular mechanism underlying cell death can potentially lead to finding a modality for targeted delivery towards tumor cells.Experimental
Reagents and instruments
All reagents were of the best commercial grade and were used without further purification. L-Valine, O-vanillin, pyrazole, dimethyl tin dichloride, disodium salt of calf thymus DNA (highly polymerized, stored at 4 °C), agarose (Sigma-Aldrich), Tris(hydroxymethyl)aminomethane, CuCl2·2H2O, anhydrous ZnCl2, (E. Merck), and supercoiled pBR322 plasmid DNA (Genei) were used as received. The Schiff base ligand was synthesized by the condensation of L-valine with O-vanillin in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio with a slight modification of the previously reported procedure.43
1 ratio with a slight modification of the previously reported procedure.43
The molar conductances were measured at room temperature with a Eutech CON 510 Conductivity Bridge. IR spectra (KBr pellets) in the range of 4000–400 cm−1 were recorded using an Interspec 2020 FTIR spectrometer. The digital polarimeter of JASCO P-1020 (Jasco International Co., Ltd., Tokyo, Japan) with a 10 cm optical length cell at 25 °C was used to measure specific rotation. 1H, 13C, and 119Sn NMR spectra were recorded using a Bruker Avance II 400 NMR spectrometer at 25 °C. The EPR spectrum was performed with a Varian E 112 spectrometer using X-band frequency (9.1 GHz) at LNT in the solid state. The magnetic moment was calculated with a Sherwood Scientific Magnetic Balance MSBAuto. Electronic spectra were recorded using a UV-1700 PharmaSpec UV-vis spectrophotometer (Shimadzu). Electrospray mass spectra were recorded with a Micromass Quattro II triple quadrupole mass spectrometer. Emission spectra were determined with a Shimadzu RF 5301 PC spectrofluorophotometer. Cleavage experiments were performed with the help of Axygen electrophoresis supported by a Genei power supply with a potential range of 50–500 volts, visualized and photographed using a Vilber-INFINITY gel documentation system. Solutions of CT-DNA in buffer gave a ratio of UV absorbance at 260 and 280 nm of ca. 1.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, indicating that the DNA was sufficiently free of protein. Absorption spectroscopy determined the DNA concentration per nucleotide with the molar absorption coefficient 6600 dm3 mol−1 cm−1 at 260 nm.44 Circular dichroism spectra of DNA were obtained using a Circular Dichroism (CD) Spectrometer with a Stop Flow-Applied PhotoPhysics Chirascan. All experiments were conducted using a 200 μL quartz cell. Each CD spectrum was collected after averaging over at least 3 accumulations using a scan speed of 100 nm min−1 and a 5 s response time. The machine and cuvette baselines were subtracted, and the resultant spectra were zeroed outside the absorption bands.
1, indicating that the DNA was sufficiently free of protein. Absorption spectroscopy determined the DNA concentration per nucleotide with the molar absorption coefficient 6600 dm3 mol−1 cm−1 at 260 nm.44 Circular dichroism spectra of DNA were obtained using a Circular Dichroism (CD) Spectrometer with a Stop Flow-Applied PhotoPhysics Chirascan. All experiments were conducted using a 200 μL quartz cell. Each CD spectrum was collected after averaging over at least 3 accumulations using a scan speed of 100 nm min−1 and a 5 s response time. The machine and cuvette baselines were subtracted, and the resultant spectra were zeroed outside the absorption bands.
Synthesis
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N imine proton), 7.57 (br, 2H, –NH, Hpz), 7.77–7.76 (d, 4H, Hpz), 7.14–7.12 (m, 2H, Hpz), 6.86–6.76 (m, 2H, phenyl ring), 6.26–6.22 (t, 1H, phenyl ring), 3.37 (s, 1H, N–CH–COO, valine), 3.10 (s, 3H, OCH3, vanillin), 2.51–2.49 (m, 1H, CH–(CH3)2, valine), 0.93–0.76 (m, 6H, CH–(CH3)2, valine). 13C NMR (100 MHz, DMSO-d6, δ): 169.17 (C
N imine proton), 7.57 (br, 2H, –NH, Hpz), 7.77–7.76 (d, 4H, Hpz), 7.14–7.12 (m, 2H, Hpz), 6.86–6.76 (m, 2H, phenyl ring), 6.26–6.22 (t, 1H, phenyl ring), 3.37 (s, 1H, N–CH–COO, valine), 3.10 (s, 3H, OCH3, vanillin), 2.51–2.49 (m, 1H, CH–(CH3)2, valine), 0.93–0.76 (m, 6H, CH–(CH3)2, valine). 13C NMR (100 MHz, DMSO-d6, δ): 169.17 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 168.52 (C
O), 168.52 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 155.85 (C–OCH3, aromatic carbon), 148.77 (C–O, aromatic carbon), 138.41, 137.72, 127.35 (Hpz, aromatic carbons), 118.25, 114.80, 113.54, 104.20, 103.32, 99.49 (phenyl ring), 76.08 (N–C–COO), 54.29 (OCH3), 48.57 (C–(CH3)2), 18.81, 17.98 (methyl carbons). ESI-MS {in DMSO, +ve, observed (calcd)}: 453.3 (453.8), [C19H23N5O4Zn + 3H+].
N), 155.85 (C–OCH3, aromatic carbon), 148.77 (C–O, aromatic carbon), 138.41, 137.72, 127.35 (Hpz, aromatic carbons), 118.25, 114.80, 113.54, 104.20, 103.32, 99.49 (phenyl ring), 76.08 (N–C–COO), 54.29 (OCH3), 48.57 (C–(CH3)2), 18.81, 17.98 (methyl carbons). ESI-MS {in DMSO, +ve, observed (calcd)}: 453.3 (453.8), [C19H23N5O4Zn + 3H+].![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, drop-wise and stirring for 4 h at ambient temperature. The green-colored product was isolated, washed with methanol and dried in vacuo. Yield: 74%. m.p. = 130 °C (decomposed). [α]25D = +29.2 (10−3 M, DMSO). ΛM (1 × 10−3 M, DMSO) = 8.8 ohm−1 cm2 mol−1 for IR (νmax/cm−1): 1628, 1551, 1358, 743, 595, 556, 486, 425. 119Sn NMR (149.19 MHz, DMSO-d6, δ): −592.61. ESI-MS {in DMSO, +ve, observed (calcd)}: 667.0 (667.8), [C21H31N5O6CuSn]·2H2O.
1 ratio, drop-wise and stirring for 4 h at ambient temperature. The green-colored product was isolated, washed with methanol and dried in vacuo. Yield: 74%. m.p. = 130 °C (decomposed). [α]25D = +29.2 (10−3 M, DMSO). ΛM (1 × 10−3 M, DMSO) = 8.8 ohm−1 cm2 mol−1 for IR (νmax/cm−1): 1628, 1551, 1358, 743, 595, 556, 486, 425. 119Sn NMR (149.19 MHz, DMSO-d6, δ): −592.61. ESI-MS {in DMSO, +ve, observed (calcd)}: 667.0 (667.8), [C21H31N5O6CuSn]·2H2O.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N imine proton), 7.53–7.51 (d, 4H, Hpz), 7.16–7.11 (m, 2H, Hpz), 6.98–6.79 (m, 3H, phenyl ring), 4.20 (s, 1H, N–CH–COO, valine), 3.77 (s, 3H, OCH3, vanillin), 2.52–2.50 (m, 1H, CH–(CH3)2, valine), 1.10 (s, 6H, –(CH3)2Sn), 1.08–0.98 (m, 6H, CH–(CH3)2, valine). 13C NMR (100 MHz, DMSO-d6, δ): 176.18 (C
N imine proton), 7.53–7.51 (d, 4H, Hpz), 7.16–7.11 (m, 2H, Hpz), 6.98–6.79 (m, 3H, phenyl ring), 4.20 (s, 1H, N–CH–COO, valine), 3.77 (s, 3H, OCH3, vanillin), 2.52–2.50 (m, 1H, CH–(CH3)2, valine), 1.10 (s, 6H, –(CH3)2Sn), 1.08–0.98 (m, 6H, CH–(CH3)2, valine). 13C NMR (100 MHz, DMSO-d6, δ): 176.18 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 175.81 (C
O), 175.81 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 156.83 (C–OCH3, aromatic carbon), 150.99 (C–O, aromatic carbon), 136.04–126.90 (Hpz, aromatic carbon), 118.34, 117.69, 117.16, 114.23, 110.45, 103.28 (phenyl ring), 70.91 (N–C–COO), 55.42 (OCH3), 34.32 [C–(CH3)2], 21.62, 19.82 (methyl carbons of valine), 18.91, 17.70 [–(CH3)2Sn]. 119Sn NMR (149.19 MHz, DMSO-d6, δ): −580.86. ESI-MS {in DMSO, +ve, observed (calcd)}: 653.1 (653.6), [C21H31N5O6ZnSn + 2H·H2O].
N), 156.83 (C–OCH3, aromatic carbon), 150.99 (C–O, aromatic carbon), 136.04–126.90 (Hpz, aromatic carbon), 118.34, 117.69, 117.16, 114.23, 110.45, 103.28 (phenyl ring), 70.91 (N–C–COO), 55.42 (OCH3), 34.32 [C–(CH3)2], 21.62, 19.82 (methyl carbons of valine), 18.91, 17.70 [–(CH3)2Sn]. 119Sn NMR (149.19 MHz, DMSO-d6, δ): −580.86. ESI-MS {in DMSO, +ve, observed (calcd)}: 653.1 (653.6), [C21H31N5O6ZnSn + 2H·H2O].DNA binding and cleavage studies
DNA binding experiments that included absorption spectral studies, fluorescence, circular dichroism, DNA cleavage and a topoisomerase inhibition assay conformed to the standard methods45–47 and practices previously adopted by our laboratory.48–50 Standard error limits were estimated using all data points.Measurement of SOD-like activity
Superoxide dismutase (SOD)-like activity was investigated using Beauchamp and Fridovich’s method, as improved by Imanari et al.51 This method is based on the inhibitory effect of SOD on the reduction of nitro blue tetrazolium (NBT) by the O2˙− generated by the xanthine/xanthine oxidase system. The assay was carried out in the assay buffer containing 50 mM Tris–HCl, pH = 8.0, 0.1 mM DTPA and 0.1 mM hypoxanthine. The radical detector consisted of a tetrazolium salt and was diluted with assay buffer. Similarly, the solutions of SOD standards and xanthine oxidase were prepared in a sample buffer consisting of 50 mM Tris–HCl, pH 8.0. All the complexes were dissolved in DMSO and the absorbance was reported for each set of concentrations at 15 minute intervals. Results were graphed as the % inhibition of NBT reduction taken at various concentrations (0.04–0.20 μM) for each of the studied complexes. The IC50 values are reported as equivalent concentrations (μM) to 1 U bovine erythrocyte superoxide dismutase (native SOD).Anticancer activity studies
The percentage inhibition was calculated from this data using the formula:
The IC50 value was determined as the concentration of the complex/cisplatin that is required to decrease the absorbance to half that of the control.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio) (Sigma Chemical Co., St. Louis, MO, USA), and a cover slip was laid over it to reduce light diffraction.53 At random, 300 cells were observed in a fluorescent microscope (Carl Zeiss, Jena, Germany) fitted with a 377–355 nm filter and examined at 400× magnification. The percentage of cells reflecting pathological changes was calculated.
1 ratio) (Sigma Chemical Co., St. Louis, MO, USA), and a cover slip was laid over it to reduce light diffraction.53 At random, 300 cells were observed in a fluorescent microscope (Carl Zeiss, Jena, Germany) fitted with a 377–355 nm filter and examined at 400× magnification. The percentage of cells reflecting pathological changes was calculated.Acknowledgements
The authors are grateful to DBT (Scheme no. BT/PR6345/MED/14/784/2005), the DST–PURSE Programme and DRS-1 (SAP) from UGC, New Delhi, India, for the financial support. Ahmad Asim is grateful to UGC for the BSR fellowship. The NMR and ESI-MS facilities of SAIF, Panjab University, Chandigarh; the Elemental Analysis facility of STIC, Cochin; the EPR studies by SAIF, IIT, Mumbai; and the CD facility of AIRF, JNU, New Delhi, are gratefully acknowledged. The Doerenkamp-Zbinden Foundation, Switzerland, is thanked for establishing the in vitro toxicology facility at MGDC, Bharathidasan University, Tiruchirappalli.References
- F. Arjmand, F. Sayeed, S. Parveen, S. Tabassum, A. S. Juvekar and S. M. Zingde, Dalton Trans., 2013, 3390 RSC.
- ACS, Global cancer facts and figures 2007, American Cancer Society, Atlanta, GA, 2007 Search PubMed.
- T. O. Callaghan, Nature, 2011, 471, S2–S4 CrossRef PubMed.
- (a) J. L. Alonso-Romero and A. Piñero-Madrona, World J. Obstet. Gynecol., 2013, 2, 21 CrossRef; (b) S. Bhattacharyya and M. Dixit, Dalton Trans., 2011, 6112 RSC.
- I. Vergote, C. G. Trope, F. Amant, G. B. Kristensen, T. Ehlen, N. Johnson and N. S. Reed, N. Engl. J. Med., 2010, 363, 943 CrossRef CAS PubMed.
- World Health Organization, World Cancer Report 2014, 2014, Ch. 1.1, ISBN 9283204298 Search PubMed.
- V. Laquintana, A. Trapani, N. Denora, F. Wang, J. M. Gallo and G. Trapani, Expert Opin. Drug Delivery, 2009, 6, 1017 CrossRef CAS PubMed.
- A. Hordyjewska, L. Popiołek and J. Kocot, BioMetals, 2014, 27, 611 CrossRef CAS PubMed.
- G. M. Tozer, C. Kanthou, G. Lewis, V. E. Prise, B. Vojnovic and S. A. Hill, Br. J. Radiol., 2008, 81, S12–S20 CrossRef CAS PubMed.
- K. G. Daniel, D. Chen, S. Orlu, Q. C. Cui, F. R. Miller and Q. P. Dou, Breast Cancer Res., 2005, 7, R897 CrossRef CAS PubMed.
- (a) Metallotherapeutic Drugs and Metal Based Diagnostic Agents. The Use of Metals in Medicine, ed. M. Gielen and E. R. T. Tiekink, J. Wiley & Sons, 2005, pp. 421–439 Search PubMed; (b) R. A. Khan, S. Yadav, Z. Hussain, F. Arjmand and S. Tabassum, Dalton Trans., 2014, 2534 RSC.
- M. Nath and P. K. Saini, Dalton Trans., 2011, 7077 RSC.
- (a) A. Terenzi, R. Bonsignore, A. Spinello, C. Gentile, A. Martorana, C. Ducani, B. Högberg, A. M. Almerico, A. Lauria and G. Barone, RSC Adv., 2014, 4, 33245 RSC; (b) E. Sundaravadivel, S. Vedavalli, M. Kandaswamy, B. Varghese and P. Madankumar, RSC Adv., 2014, 4, 40763 RSC; (c) F. Arjmand, M. Muddassir, Y. Zaidi and D. Ray, Med. Chem. Commun., 2013, 4, 394 RSC; (d) Y. Zaidi, F. Arjmand, N. Zaidi, J. A. Usmani, H. Zubair, K. Akhtar, M. Hossain and G. G. H. A. Shadab, Metallomics, 2014, 6, 1469 RSC.
- S. Bhattacharyya, D. Ghosh, A. Endo, K. Shimizu, T. J. R. Weakley and M. Chaudhury, J. Chem. Soc., Dalton Trans., 1999, 3859 RSC.
- J. C. Pessoa, M. J. Calhorda, I. Cavaco, P. J. Costa, I. Correia, D. Costa, L. F. Vilas-Boas, V. Félix, R. D. Gillard, R. T. Henriquesa and R. Wiggins, Dalton Trans., 2004, 2855 RSC.
- S. Tabassum, S. Amir, F. Arjmand, C. Pettinari, F. Marchetti, N. Masciocchi, G. Lupidi and R. Pettinari, Eur. J. Med. Chem., 2013, 60, 216 CrossRef CAS PubMed.
- (a) F. Marchetti, C. Pettinari, A. Cingolani, R. Pettinari, M. Rossi and F. Caruso, J. Organomet. Chem., 2002, 64, 5134 Search PubMed; (b) M. A. Affan, Y. Z. Liew, F. B. Ahmad, M. B. Shamsuddin and B. M. Yamin, Indian J. Chem., Sect. A: Inorg., Phys., Theor. Anal., 2007, 46, 1063 Search PubMed.
- F. Caruso, D. Leonesi, F. Marchetti, E. Rivarola, M. Rossi, V. Tomov and C. Pettinari, J. Organomet. Chem., 1996, 51, 929 Search PubMed.
- C. Pettinari, F. Caruso, N. Zaffaroni, R. Villa, F. Marchetti, R. Pettinari, C. Phillips, J. Tanski and M. Rossi, J. Inorg. Biochem., 2006, 100, 58 CrossRef CAS PubMed.
- (a) P. F. Rapheal, E. Manoj and M. R. P. Kurup, Polyhedron, 2007, 26, 818 CrossRef CAS; (b) A. C. Mot, S. A. Syrbu, S. V. Makarov, G. Damian and R. S. Dumitrescu, Inorg. Chem. Commun., 2012, 18, 1 CrossRef CAS.
- (a) E. Kikuta, M. Murata, N. Katsube, T. Koike and E. Kimura, J. Am. Chem. Soc., 1999, 121, 5426 CrossRef CAS; (b) R. S. Kumar and S. Arunachalam, Eur. J. Med. Chem., 2009, 44, 1878 CrossRef CAS PubMed; (c) S. Tabassum, R. A. Khan, F. Arjmand, A. S. Juvekar and S. M. Zingde, Eur. J. Med. Chem., 2010, 45, 4797 CrossRef CAS PubMed; (d) M. Chauhan and F. Arjmand, J. Organomet. Chem., 2007, 692, 5156 CrossRef CAS.
- (a) X. Dong, X. Wang, M. Lin, H. Sun, X. Yang and Z. Guo, Inorg. Chem., 2010, 49, 2541 CrossRef CAS PubMed; (b) A. Jain, J. Wang, E. R. Mashack, B. S. J. Winkel and K. J. Brewer, Inorg. Chem., 2009, 48, 9077 CrossRef CAS PubMed; (c) P. de Hoog, C. Boldron, P. Gamez, K. Sliedregt-Bol, I. Roland, M. Pitie, R. Kiss, B. Meunier and J. Reedijk, J. Med. Chem., 2007, 50, 3148 CrossRef CAS PubMed.
- F. Arjmand, A. Jamsheera, M. Afzal and S. Tabassum, Chirality, 2012, 24, 977 CrossRef CAS PubMed.
- C. Tong, Z. Hu and W. Liu, J. Agric. Food Chem., 2005, 53, 6207 CrossRef CAS PubMed.
- G. Han and P. Yang, J. Inorg. Biochem., 2002, 91, 230 CrossRef CAS PubMed.
- K. Karidi, A. Garoufis, A. Tsipis, N. Hadjiliadis, H. den Dulk and J. Reedijk, Dalton Trans., 2005, 1176 RSC.
- P. T. Selvi and M. Palaniandavar, Inorg. Chim. Acta, 2002, 337, 420 CrossRef CAS.
- P. Huang, L. Feng, E. A. Oldham, M. J. Keating and W. Plunkett, Nature, 2000, 407, 390 CrossRef CAS PubMed.
- G. Tabbi, W. L. Driessen, J. Reedijk, R. P. Bonomo, N. Veldman and A. L. Spek, Inorg. Chem., 1997, 36, 1168 CrossRef CAS PubMed.
- J. E. Weder, C. T. Dillon, T. W. Hambley, B. J. Kennedy, P. A. Laya, J. R. Biffin, H. L. Regtop and N. M. Davies, Coord. Chem. Rev., 2002, 232, 95 CrossRef CAS.
- (a) C. A. Detmer III, F. V. Pamatong and J. R. Bocarsly, Inorg. Chem., 1997, 36, 3676 CrossRef PubMed; (b) M. S. Melvin, M. W. Calcutt, R. E. Noftle and R. A. Manderville, Chem. Res. Toxicol., 2002, 15, 742 CrossRef CAS PubMed.
- C. J. Burrows and J. G. Muller, Chem. Rev., 1998, 98, 1109 CrossRef CAS PubMed.
- B. Selvakumar, V. Rajendiran, P. U. Maheswari, H. S. Evans and M. Palaniandavar, J. Inorg. Biochem., 2006, 100, 316 CrossRef CAS PubMed.
- L. F. Chin, S. M. Kong, H. L. Seng, K. S. Khoo, R. Vikneswaran, S. G. Teoh, M. Ahmad, S. B. A. Khoo, M. J. Maah and C. H. Ng, J. Inorg. Biochem., 2011, 105, 339 CrossRef CAS PubMed.
- B. Montaner, W. C. Ávila, M. Martinell, R. Öllinger, J. Aymami, E. Giralt and R. P. Tomas, Toxicol. Sci., 2005, 85, 870 CrossRef CAS PubMed.
- K. Suzuki and M. Uyeda, Biosci., Biotechnol., Biochem., 2002, 66, 1706 CrossRef CAS PubMed.
- K. Suzuki, F. Shono and M. Uyeda, Biosci., Biotechnol., Biochem., 1998, 62, 2073 CrossRef CAS PubMed.
- B. Bielawski, A. Bielawski, T. Anchim and S. Wolczynski, Biol. Pharm. Bull., 2005, 28, 1004 Search PubMed.
- S. A. S. Sakinah, S. T. Handayani and L. P. A. Hawariah, Cancer Cell Int., 2007, 7, 1 CrossRef PubMed.
- R. S. Wong, J. Exp. Clin. Cancer Res., 2011, 30, 87 CrossRef CAS PubMed.
- K. Choi, J. Kim, G. W. Kim and C. Choi, Curr. Neurovasc. Res., 2009, 6, 213 CrossRef CAS PubMed.
- M. V. Jain, A. M. Paczulla, T. Klonisch, F. N. Dimgba, S. B. Rao, K. Roberg, F. Schweizer, C. Lengerke, P. Davoodpour, V. R. Palicharla, S. Maddika and M. Łos, J. Cell. Mol. Med., 2013, 17, 12 CrossRef CAS PubMed.
- (a) G. Wang and J. C. Chang, Synth. React. Inorg. Met.-Org. Chem., 1994, 24, 623 CrossRef CAS; (b) S. Tabassum, A. Asim, R. A. Khan, Z. Hussain, S. Srivastav, S. Srikrishna and F. Arjmand, Dalton Trans., 2013, 16749 RSC.
- J. Marmur, J. Mol. Biol., 1961, 3, 208 CrossRef CAS.
- M. E. Reichmann, S. A. Rice, C. A. Thomas and P. Doty, J. Am. Chem. Soc., 1954, 76, 3047 CrossRef CAS.
- A. Wolfe, G. H. Shimer and T. Meehan, Biochemistry, 1987, 26, 6392 CrossRef CAS PubMed.
- J. R. Lakowicz and G. Weber, Biochemistry, 1973, 12, 4171 CrossRef CAS PubMed.
- (a) S. Tabassum, M. Zaki, M. Afzal and F. Arjmand, Dalton Trans., 2013, 10029 RSC; (b) S. Tabassum, A. Asim, F. Arjmand, M. Afzal and V. Bagchi, Eur. J. Med. Chem., 2012, 58, 308 CrossRef CAS PubMed; (c) S. Tabassum, W. M. Al-Asbahy, M. Afzal, F. Arjmand and V. Bagchi, Dalton Trans., 2012, 4955 RSC.
- (a) R. A Khan, A. Asim, R. Kakkar, D. Gupta, V. Bagchi, F. Arjmand and S. Tabassum, Organometallics, 2013, 32, 2546 CrossRef; (b) M. Chauhan, K. Banerjee and F. Arjmand, Inorg. Chem., 2007, 30, 3072 CrossRef PubMed.
- (a) F. Arjmand and M. Muddassir, Chirality, 2011, 23, 250 CrossRef CAS PubMed; (b) S. Tabassum, S. Mathur, F. Arjmand, K. Mishra and K. Banerjee, Metallomics, 2012, 4, 205 RSC.
- C. Beauchamp and I. Fridovich, Anal. Biochem., 1971, 44, 276–287 CrossRef CAS PubMed.
- T. Mosmann, J. Immunol. Methods, 1983, 65, 55 CrossRef CAS PubMed.
- D. L. Spector, R. D. Goldmann and L. A. Leinwand, Cell: A Laboratory Manual; Culture and Biochemical Analysis of Cells, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, 1998, vol. 1, pp. 34.1–34.9 Search PubMed.
- S. Salvioli, A. Ardizzonim, C. Franceschi and A. Cossarizza, FEBS Lett., 1997, 411, 77 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: The ESI-MS, EPR, UV-vis, fluorescence spectra of the complexes (Fig. S1–S6). See DOI: 10.1039/c5ra07333b |
| This journal is © The Royal Society of Chemistry 2015 |