KOt-Bu/DMF promoted intramolecular cyclization of 1,1′-biphenyl aldehydes and ketones: an efficient synthesis of phenanthrenes†
Abstract
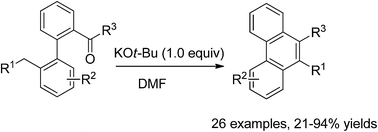
A new synthesis of phenanthrene derivatives has been achieved through intramolecular cyclization of 1,1′-biphenyl aldehydes and ketones promoted by KOt-Bu/DMF. A free radical reaction pathway is proposed.

 Please wait while we load your content...
Please wait while we load your content...