Noble metal clusters protected with mixed proteins exhibit intense photoluminescence†
Jyoti Sarita Mohanty‡
a,
Ananya Baksi‡a,
Haiwon Leeb and
T. Pradeep*a
aDST Unit of Nanoscience (DST UNS), Thematic Unit of Excellence (TUE), Department of Chemistry, Indian Institute of Technology Madras, Chennai – 600 036, India. E-mail: Pradeep@iitm.ac.in; Fax: +91-44-2257-0545
bDepartment of Chemistry, Institute of Nanoscience and Technology, Hanyang University, Seoul 133-791, Korea
First published on 8th May 2015
Abstract
In this paper is reported the synthesis and detailed mass spectrometric and spectroscopic characterization of highly luminescent Au and Ag clusters protected with mixed proteins. Taking advantage of the aggregation tendency of the protein, lysozyme (Lyz), an inter-protein conjugate was made from a physical mixture of two proteins, bovine serum albumin (BSA) and Lyz. Based on matrix-assisted laser desorption/ionization mass spectrometry data, the new cluster is assigned as ∼Au36@BSA–Lyz. This specific system showed a very high red luminescence and the calculated quantum yield was 42% which is the highest to date for such cluster systems. A similar study on an Ag system showed the formation of ∼Ag35@BSA–Lyz when a similar metal and protein concentration was used. By varying the concentration of the Ag precursor, different compositions of the cluster protected by the mixed protein were achieved. Such a system with a high quantum yield can be used for various applications such as sensors for ultralow levels of analytes, fluorescent tags and for tracking biomolecules in real systems.
1. Introduction
Noble metal nanoclusters are one of the most fascinating areas of research in contemporary materials science.1–5 Several metals have been used in this context among which gold (Au) and silver (Ag) have drawn the most attention. Most cluster chemistry is concerned with monolayer protected clusters. Thiol ligands are of greater interest as the Au–S bond is strong and by changing the ligand (thiol), the properties of the clusters can be altered. Many thiol protected Au and Ag clusters have been reported and some of them have been crystallized. Examples include: Au25(SR)18,6,7 Au30S(SR)18,8 Au38(SR)24,9 Au36(SR)24,10 Au102(SR)44,11 and Ag44(SR)30.12,13 Three types of modifications are possible on such monolayer protected clusters: (i) ligand exchange,14–16 (ii) alloying12 of the core and (iii) alloying and ligand exchange simultaneously. Both of these have been studied in detail for monolayer protected Au clusters. Some of these chemically modified clusters have been crystallized.12Macromolecular templates are another platform for synthesizing such clusters.4,17,18 DNA,19 dendrimers20 and most recently proteins21 have been used as templates for cluster formation. The most often used proteins are bovine serum albumin (BSA),21–28 human serum albumin,29,30 lactoferrin (Lf),31,32 lysozyme (Lyz),33–36 insulin,37 and so on. Major characterization methods of such clusters involve mass spectrometry (MS), but matrix-assisted laser desorption/ionization (MALDI) is mainly used,21,25,27–29,31,34,38 as none of these clusters could be crystallized so far. The general synthetic route of such clusters involves adduct formation between the protein and the metal and subsequent reduction of the ion to the M(0) state at an elevated pH.21,31 During the core formation, inter-protein metal ion transfer takes place which leads to the regeneration of the free protein. As the core evolves to a bigger size, more and more free protein regeneration can be seen.31 A variety of cores have been reported, depending on the size and structure of the protein.31,34 For smaller proteins such as Lyz, core compositions have been confirmed from the aggregates (formed via inter-protein interaction through a salt bridge).34 For a single protein, by changing the experimental conditions and using different parameters, one can modify the core size as shown in a recent study by predefining the protein structure at a specific pH.38 Different methods have been tried for modifying the core and shell composition such as core etching,23 a sonochemical method,39 microwave synthesis,40,41 slow reduction by carbon monoxide,38 and so on. Such clusters exhibit intense luminescence which is highly sensitive to the presence of foreign elements which can interact and quench the luminescence.2 This luminescence property has been applied for sensing metal ions24,26,33,40 and small molecules,30,42–48 as well as in vitro and in vivo imaging and labelling.23,49 A few of the proteins are known to retain biological activity even after cluster formation,37 indicating that the protein remains as it was and the cluster formation does not occur via the involvement of the active site of the protein. The presence of another protein might help in enhancing the Förster resonance energy transfer (FRET) probability and thus, the quantum yield may be increased. This could possibly be following the ligand exchange procedure used for monolayer protected clusters where after ligand exchange, properties of both the ligands can be monitored. Niihori et al. have separated all the ligand exchange products of Au25(SR1)18−x(SR2)x (x = 0, 1, 2…18) by chromatography and characterized them using MS.16 The supramolecular chemistry of such clusters via ligand exchange by thiolated calixerene has been studied in detail.14 Taking advantage of ligand and cyclodextrin inclusion complex formation, Mathew et al. have recently shown that supramolecular chemistry is possible for such Au25(SR)18 clusters.15 Exchanging the core with other metals can also enhance the physico-optical properties significantly as shown by Wang et al., where they have exchanged Ag with Au and obtained 200-fold increases in the luminescence (41% quantum yield).50
There are reports of ligand exchange and mixed ligand protection for monolayer protected clusters. Recently, ligand exchange of Au25(SR)18 was separated using high-performance liquid chromatography and each of the exchanged products were observed using MALDI-MS.16 Although a mixed protein matrix such as egg shell membrane46 and hair fibre44 were used for cluster synthesis, none of these clusters could be characterized to get an idea about their precise core size. Proteins are macromolecules and they behave differently to small thiol ligands. Protein–protein interaction has been studied by biologists and this has strong biological implications. This kind of interaction is very specific and occurs only between specific proteins. Following the ligand exchange of monolayer protected clusters, the effect of addition of an external protein on a preformed protein protected cluster is reported in this paper.
Most of the previous reports have claimed that the luminescence of protein protected clusters is because of FRET between the protein and the cluster.4,32 Compared to monolayer protected Au clusters, protein protected clusters have a higher quantum yield but the yield is still not very promising unlike that obtained for the fluorescent dyes (15% for protein protected clusters whereas dyes show more than 95%). Keeping this in mind, an attempt to ligand exchange protein protected clusters was made, where it was hoped to see different properties by protein exchange. More interestingly, we expected to be in a position to regulate the properties of the proteins as well as the cluster core.
In this paper is reported the formation of highly fluorescent Au quantum clusters with the highest quantum yield (42.4%) in a mixed protein system. Two differently sized proteins, namely BSA and Lyz, were used for this study and the reactants as well as the products were probed using MS and optical spectroscopic techniques. A new cluster core, Au36, protected with both BSA and Lyz was obtained with a four-fold increase in the fluorescence intensity which leads to a 42.4% quantum yield for this system.
2. Experimental
2.1. Reagents and materials
Bovine serum albumin at pH 6–7 was purchased from SRL Chemical Co. Ltd., India. Lysozyme and sinapic acid were purchased from Sigma-Aldrich. Tetrachloroauric acid trihydrate (HAuCl4·3H2O) was purchased from CDH, India. Silver nitrate and sodium hydroxide (NaOH) were purchased from Rankem, India. Sodium borohydride (NaBH4) was purchased from Spectrochem, India. All the chemicals were used without further purification. Milli-Q water was used for all the experiments.2.2. Instrumentation
Luminescence measurements were carried out using a Jobin Yvon NanoLog spectrofluorometer. Both the excitation and emission spectra were collected with a band pass of 3 nm. MALDI-MS studies were performed using an Applied Biosystems Voyager-DE PRO Biospectrometry Workstation. A pulsed nitrogen laser of 337 nm was used for the MALDI-MS studies. Mass spectra were collected in linear positive mode and an average of 250 shots was used for each spectrum. High resolution transmission electron microscopy (HRTEM) was performed on a Jeol JFD 3010, a 300 kV instrument, equipped with an ultra high resolution pole piece. The samples for HRTEM were prepared by dropping the dispersion on a carbon coated copper grid. Scanning electron microscopy (SEM) and energy dispersive analysis of the X-ray (EDAX) images were carried out using an FEI QUANTA 200 SEM. Samples were spotted on an indium tin oxide conducting glass substrate and dried in ambient conditions prior to SEM and EDAX measurements. X-ray photoelectron spectroscopy (XPS) studies were carried out using an Omicron ESCA probe spectrometer with polychromatic Mg Kα X-rays (hv = 1253.6 eV). The samples were spotted as drop cast films on a sample stub.2.3. Synthesis
BSA and Lyz were mixed in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio and stirred for 10 min. To this 1 mL of 6 mM HAuCl4 was added. The solution was stirred for another 15 min. Then 100 μL of 1 M NaOH was added to this mixture, and it was stirred further for 12 h to get a clear brown coloured solution. All the samples were taken directly from the reaction mixture for MALDI-MS and other characterization studies. Silver clusters were prepared keeping the protein and Ag concentration exactly the same as those used for the Au cluster synthesis. In this case, NaBH4 was used as the external reducing agent.
1 molar ratio and stirred for 10 min. To this 1 mL of 6 mM HAuCl4 was added. The solution was stirred for another 15 min. Then 100 μL of 1 M NaOH was added to this mixture, and it was stirred further for 12 h to get a clear brown coloured solution. All the samples were taken directly from the reaction mixture for MALDI-MS and other characterization studies. Silver clusters were prepared keeping the protein and Ag concentration exactly the same as those used for the Au cluster synthesis. In this case, NaBH4 was used as the external reducing agent.
3. Results and discussion
3.1. Gold cluster formation in a mixed protein matrix
Monolayer as well as protein protected Au and Ag clusters were studied extensively.1,3,17 Protein protected clusters were typically synthesized by mixing a preferred ratio of protein and metal ions to form a metal bound protein adduct and followed by reduction of the adducts at a basic condition (pH 12). At this pH, the protein unfolds [seen in circular dichroism]27,31,32,34 and disulfide bonds between the cysteine residues break. In a system containing two different proteins, the inter-protein disulfide bonds may create new inter-protein adducts. In these reactions there are a number of different types of possibilities and these are: (i) large protein-large protein, (ii) large protein-small protein and (iii) small protein-small protein. In this paper, the first two possibilities were studied. Lf, a large protein with a mass of 83 kDa and Lyz, a small protein with a mass of 14.3 kDa were chosen in combination with BSA to study the exchange.Isolated Au and Ag clusters protected with BSA25,27 as well as Lyz34 were characterized thoroughly using several spectroscopic and mass spectrometric tools. They show well defined mass spectral signatures as observed from MALDI-MS analysis. The core size of such clusters is assigned by considering the mass shift from the parent protein after cluster formation. In most of the cases, core size depends on the concentration of protein and metal ions in the solution and varies linearly with metal concentration. At a specific Au3+ concentration, ∼Au30@BSA27 forms (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 BSA
16 BSA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Au3+ molar concentration), whereas an Au10 core is observed for Lyz (1
Au3+ molar concentration), whereas an Au10 core is observed for Lyz (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 Lyz
4 Lyz![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Au3+ molar concentration).34
Au3+ molar concentration).34
A physical mixture of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
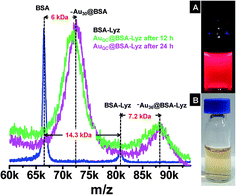
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (75 μM) BSA and Lyz was used and the mixture was incubated in the presence of 6 mM Au3+ for a few minutes to allow reduction of Au3+ to Au+ by the aromatic amino acids of the proteins and subsequently NaOH was added. Formation of clusters was confirmed from the appearance of a slight red luminescence under ultraviolet (UV) light after 4 h of incubation. The mixture was further incubated for up to 12 h to allow the complete conversion of Au+ to Au0. Fig. 1 shows the comparison of the MALDI-MS spectra of BSA–Lyz and AuQC@BSA–Lyz in linear positive ion mode. In the 60–100 kDa mass range, the BSA–Lyz mixture showed three peaks corresponding to BSA+, BSA–Lyz+ and BSA–Lyz2+ (see next for details). During cluster formation, a new peak appeared at 88.3 kDa together with ∼Au30@BSA at 72 kDa (see (ESI) Fig. S1† for the MALDI-MS of ∼Au30@BSA). A corresponding +2 charged species was observed at 44.1 kDa. If it is assumed that there was adduct formation between the proteins, the new peak can be assigned as ∼Au36@BSA–Lyz. The peak position remained the same after 24 h as well as at 48 h of incubation indicating the formation of a stable species in the solution. The possibility of formation of Au36 in monolayer protected clusters was reported previously.10 In the lower mass region (<20 kDa), a few Au attachments were observed with Lyz together with the fragments of BSA and Lyz (see ESI Fig. S2†).
1 (75 μM) BSA and Lyz was used and the mixture was incubated in the presence of 6 mM Au3+ for a few minutes to allow reduction of Au3+ to Au+ by the aromatic amino acids of the proteins and subsequently NaOH was added. Formation of clusters was confirmed from the appearance of a slight red luminescence under ultraviolet (UV) light after 4 h of incubation. The mixture was further incubated for up to 12 h to allow the complete conversion of Au+ to Au0. Fig. 1 shows the comparison of the MALDI-MS spectra of BSA–Lyz and AuQC@BSA–Lyz in linear positive ion mode. In the 60–100 kDa mass range, the BSA–Lyz mixture showed three peaks corresponding to BSA+, BSA–Lyz+ and BSA–Lyz2+ (see next for details). During cluster formation, a new peak appeared at 88.3 kDa together with ∼Au30@BSA at 72 kDa (see (ESI) Fig. S1† for the MALDI-MS of ∼Au30@BSA). A corresponding +2 charged species was observed at 44.1 kDa. If it is assumed that there was adduct formation between the proteins, the new peak can be assigned as ∼Au36@BSA–Lyz. The peak position remained the same after 24 h as well as at 48 h of incubation indicating the formation of a stable species in the solution. The possibility of formation of Au36 in monolayer protected clusters was reported previously.10 In the lower mass region (<20 kDa), a few Au attachments were observed with Lyz together with the fragments of BSA and Lyz (see ESI Fig. S2†).
3.2. Identification of mixed protein aggregates by MALDI-MS
In order to check the formation of BSA–Lyz adducts in specific reaction conditions, several control experiments were performed and the presence of this species was verified using MALDI-MS. The mass spectra of: (i) pure BSA, (ii) Au+–BSA adduct, (iii) Lyz, (iv) Au+–Lyz adduct, (v) BSA and Lyz mixture in presence of NaOH and (vi) BSA and Lyz mixture, are shown in Fig. 2. The molecular ion peak of BSA appears at 66.7 kDa as shown in spectrum (i). Corresponding a doubly charged species at 33.3 kDa was also observed. Beyond 66.7 kDa, only the dimer of BSA was observed at 133.4 kDa and no peak was observed in the mass range of 70–100 kDa. Once Au3+ was added to the BSA solution, the main protein peak was shifted by 3.2 kDa as shown in (ii) and the resulting adduct was assigned to ∼Au16–BSA. In this state, Au is in the +1 oxidation state (as revealed by XPS).31 The monomer of Lyz shows a peak at m/z 14.3 kDa and the corresponding doubly charged species was observed at m/z 7.2 kDa. Lyz is known to form aggregates in solution and a maximum aggregate of a hexamer of individual protein was observed in the mass range studied. The corresponding dimer, trimer and tetramer peaks were observed at m/z 28.6, 42.9 and 57.2 kDa, respectively (see ESI Fig. S3†). Pentamers and hexamers were observed at m/z 71.5 and 85.8 kDa, respectively, as shown in (iii). Beyond this the peak intensity was not significant. The Au+–Lyz adduct showed multiple Au attachments to the protein monomer as well as to the aggregates. The peaks were separated by a peak at m/z 197 which was because of Au and a maximum of up to 10 Au attachments to Lyz were observed (see ESI Fig. S4†). In the lower mass range, gas phase bare clusters were observed, which were reported previously.35 When BSA was mixed with Lyz, several possibilities exist such as Lyzm (m = 1, 2, 3, 4), BSAn (n = 1, 2) and LyzmBSAn (m = 1, 2; n = 1, 2). All these species were identified using MS in the higher mass region (m/z 80–150 kDa). Two distinct peaks were observed at m/z 81.0 kDa and 95.3 kDa, which appear to be spaced successively at 14.3 kDa from the BSA peak (m/z 66.7 kDa). A similar 14.3 kDa separation was observed in the BSA dimer region (see ESI Fig. S5†) beyond which, because of poor resolution and reduced intensity, other peaks could not be resolved. This separation clearly indicates the possibility of adduct formation between BSA and Lyz. This adduct formation can be explained in terms of the aggregation tendency of Lyz. As was mentioned earlier, Lyz forms aggregates and those can be seen in both MALDI and ESI-MS spectra.34 | ||
| Fig. 2 MALDI-MS of individual proteins, mixture of both the proteins and Au conjugate of individual proteins, showing the formation of BSA–Lyzn (n = 0, 1, 2) conjugate. | ||
3.3. Spectroscopic and microscopic characterizations
The presence of Au along with carbon, oxygen, nitrogen, sulfur (S), sodium and chloride was confirmed from the SEM/EDAX spectrum. A Au![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S ratio (atomic%) of about 1
S ratio (atomic%) of about 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 was observed in this case. Elemental mapping of gold using Mα and sulphur using Kα show these elements to be uniform in the sample. The presence of clusters in both the proteins (∼Au36@BSA–Lyz) was further confirmed by XPS analysis which showed the presence of a metallic core (Fig. 3E). An Au 4f7/2 peak appears at 84.1 eV which shows the existence of a zero valent state of the metal. A similar binding energy was observed for Au25@Lf.31 A time dependent XPS study during the cluster formation and the corresponding MALDI-MS data was reported by Chaudhari et al., where they showed how a protein–metal complex converts to a zero valent state form during cluster formation and the appearance of a brown colour after the addition of the reducing agent also indicates the reduction of the metal precursor during the cluster formation.31 The S2p1/2 peak at 163.3 eV (see ESI Fig. S6†) confirmed the Au–S bonding, through the cysteine residues of the proteins. Protein protected clusters do not show well defined absorption features such as monolayer protected clusters.
2 was observed in this case. Elemental mapping of gold using Mα and sulphur using Kα show these elements to be uniform in the sample. The presence of clusters in both the proteins (∼Au36@BSA–Lyz) was further confirmed by XPS analysis which showed the presence of a metallic core (Fig. 3E). An Au 4f7/2 peak appears at 84.1 eV which shows the existence of a zero valent state of the metal. A similar binding energy was observed for Au25@Lf.31 A time dependent XPS study during the cluster formation and the corresponding MALDI-MS data was reported by Chaudhari et al., where they showed how a protein–metal complex converts to a zero valent state form during cluster formation and the appearance of a brown colour after the addition of the reducing agent also indicates the reduction of the metal precursor during the cluster formation.31 The S2p1/2 peak at 163.3 eV (see ESI Fig. S6†) confirmed the Au–S bonding, through the cysteine residues of the proteins. Protein protected clusters do not show well defined absorption features such as monolayer protected clusters.
Although the ultraviolet-visible (UV-Vis) technique is not useful for determining the size of the cluster in protein systems, it does help to rule out the possibility of the presence of plasmonic nanoparticles in the solution. UV-Vis spectra show a peak at ∼280 nm which is because of the presence of aromatic amino acid groups in protein systems (see ESI Fig. S7†). In the case of ∼Au36@BSA–Lyz, a similar type of absorption feature was observed at 280 nm. In addition, a broad hump was observed at 510 nm which had not been observed before for any such protein protected clusters.
HRTEM analysis of the new cluster showed the core size to be about 1.2 nm as shown in Fig. 4A. However, investigation of the cluster size using HRTEM analysis is not accurate as the electron beam induces the growth of clusters in such soft materials. But this technique helps to determine the approximate size of the samples. Because this is a high resolution instrument with point to point resolution of 0.12 nm, the approximate size is 1.2 ± 0.1 nm. In the present study, this technique showed the absence of bigger plasmonic nanoparticles in the sample. It is important to note that the size of the new species in both these proteins (∼Au36@BSA–Lyz) is similar as in the case of individual protein protected clusters. No bigger nanoparticle was found using TEM. Absence of a plasmonic peak in the absorption spectrum also supported the HRTEM data. The photographs of ∼Au36@BSA–Lyz together with individual protein protected clusters under UV as well as visible light are shown in Fig. 4B.
3.4. Enhanced photoluminescence and mechanism of cluster formation
The photoluminescence spectra of the parent clusters together with ∼Au36@BSA–Lyz are shown Fig. 4C. The emission intensity of ∼Au36@BSA–Lyz showed three- and four-fold enhancement when compared to the individual Au10@Lyz and ∼Au30@BSA, respectively, when all the clusters were excited at 365 nm keeping the overall protein concentration the same for all the cases. The calculated quantum yield of ∼Au36@BSA–Lyz was 42.4% considering rhodamine 6G as the reference, which is extremely high compared to all the reported Au clusters in various proteins. Under similar experimental conditions, the quantum yield of AuQCS@BSA and AuQC@Lyz were found to be 10.0% and 15.0%, respectively. These values were comparable to the ones reported previously.34 Like other protein protected clusters, two excitation peaks were observed in the present study, one because of the cluster core (500 nm) and the other because of the protein (365 nm). Both the excitation maxima resulted in the same emission but with different intensities. A higher intensity was observed with 365 nm excitation which was explained in terms of FRET. Muhammed et al. have shown metal enhanced luminescence of AuQC@BSA in the presence of Ag nanoparticles where the protein shell acts as a spacer between the cluster and the Ag nanoparticles.23 The distance between the fluorophore and the metal surface plays an important role in luminescence enhancement. Aggregation induced enhanced luminescence of Au(I) thiolate has been reported by Luo et al., where they have shown that the Au(I) thiolate shell surrounding the Au(0) core drastically increased the luminescence.51 Wang et al. have shown a 200-fold increase in luminescence by replacing Ag atoms with an Au cluster.50 In this case, increased emission intensity can be explained by considering the possibility of a FRET between the cluster core and the two proteins. As discussed earlier (in the section on MS), the cluster core is protected by two proteins which implies that both the proteins are in proximity for energy transfer and thus, enhancement in FRET occurs. Here the protein can be a FRET donor and the cluster can be the acceptor. In order to know whether the excess protein can also increase FRET activity, different volumes of BSA were added to ∼Au30@BSA solution. No significant increase in photoluminescent (PL) intensity was observed confirming that inter-protein energy transfer is not efficient in this case. Similar observations were also made for AuQC@Lyz. Time dependent PL did not exhibit any change in the position of the emission.As shown in Fig. 1, mixed aggregates of BSA and Lyz are also capable of forming clusters and the cluster was assigned as ∼Au36@BSA–Lyz. In one possibility, the cluster can form inside one protein (namely BSA, as it is large and can accommodate the 36 atom core) and the other protein, Lyz forms an aggregate with the as-formed BSA protected cluster. Another possibility could be the formation of the cluster in the solution and then subsequent protection by both the proteins. In the case of a mixture of proteins in the Lyz region, only 5–6 Au separations from the parent protein peak was observed, while in the BSA region, the Au30@BSA species was seen. However, another peak observed at 88.2 kDa could be because of the separation of 36 Au from the BSA–Lyz adduct. To understand the mechanism of formation, different volumes of Lyz were added to ∼Au30@BSA and the change was monitored using MS. After addition, each sample was stirred for 6 h and spotted for MALDI-MS. In the Lyz region, only the intensity enhancement with increase in Lyz concentration was observed but no cluster formation occurred in this situation. In the BSA region, a decrease in core nuclearity with the addition of Lyz was observed in the system. Two humps separated by 5 Au and 16 Au from the parent BSA (initially the separation was 30 Au) together with the appearance of free BSA were observed. The peak positions were the same for all the concentrations of Lyz used. New humps corresponding to BSA–Lyz, Au8@BSA–Lyz and ∼Au23@BSA–Lyz were observed. However, because of poor intensity, peak assignments may vary slightly (Fig. 5).
In this study, large protein-large protein interaction was also considered. For this, Lf with an m/z of 83.0 kDa was chosen. Gold clusters protected by BSA as well as by Lf have already been reported and have been are well characterized using MS. AuQC@BSA27 and AuQC@Lf32 were synthesized according to a previously reported method. When both AuQC@BSA and AuQC@Lf were mixed together, maintaining certain conditions, no distinct features were observed other than the individual cluster peak in the whole MS range. Different control experiments were also carried out (as mentioned previously) to check the interaction of both the proteins separately and those interactions with the protein protected clusters. But in none of the cases were new features observed which could be because of the bulky nature of the proteins, which restricts them to form inter-protein adducts.
3.5. Silver clusters
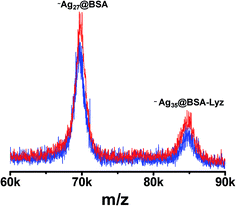
Similar studies were also performed for Ag clusters where concentration dependent growth in the cluster core was observed. For a similar concentration of Ag precursor, ∼Ag28@BSA and ∼Ag35@BSA–Lyz were observed. In the Lyz region, 8 Ag attachments to Lyz were observed. A concentration-dependent study was performed by varying the Ag concentration (final concentrations were 2, 3 and 4 mM) and keeping the protein concentration the same. ∼Ag16@BSA, ∼Ag28@BSA and ∼Ag34@BSA were observed in the BSA region, for 2, 3 and 4 mM Ag concentrations, respectively (Fig. 6).A similar mass shift with respect to Ag concentration was also reflected in the mixed protein adduct mass region where ∼Ag25@BSA–Lyz, ∼Ag35@BSA–Lyz and ∼Ag42@BSA–Lyz were observed for concentrations of 2, 3 and 4 mM Ag, respectively (see ESI Fig. S8†). XPS analysis of this system also showed the presence of a metallic Ag core where Ag is in the Ag0 state. Also, in this case, increased emission intensity comparable with Au was observed.
By comparing the data obtained for Au and Ag, it can be seen that the mass shift is always more for the mixed protein than for the individual protein. For similar concentrations of Au and Ag, Au30 and Ag26 cores can be achieved, protected by BSA alone. The other peak (at a higher mass range) was shifted by 36 Au and 35 Ag from the combined mass of BSA and Lyz. If the probabilities mentioned previously are narrowed down, only two possibilities exist: (i) ∼Au30/Ag27@BSA + Au6/Ag8@Lyz or (ii) ∼Au36/Ag35@BSA + Lyz. Considering the PL data together with the MS findings, a four-fold enhancement in emission intensity (in the case of Au) was observed which can be attributed to the presence of multiple cluster cores in the system which increases the probability of FRET. In the other case, where the presence of a single core protected by multiple protein is considered, proximity of two different proteins attached to a single core can also enhance FRET efficiently. From the TEM, pairs of clusters separated by a couple of nanometers are not seen, which would support the presence of a single cluster core, while a consequent decrease in the nuclearity of the cluster appears to suggest the idea of the presence of a multiple core. Thus, the mechanism requires further investigation which can be resolved only by crystallization of all the species.
4. Summary and conclusions
In summary, the synthesis of Au clusters protected by mixed protein systems with high quantum yield has been demonstrated. Such clusters were prepared by mixing Au3+ with a mixture of both the proteins which were then subsequently reduced to Au0 at pH 12. Using MALDI-MS, the cluster was assigned as ∼Au36@BSA–Lyz. Similar observations were also made for the Ag system, ∼Ag35@BSA–Lyz. The existence of mixed proteins in the form of an adduct was confirmed by extensive MS investigations and control studies. XPS analysis revealed that the Au cluster protected by the mixed protein system is in zero valent state. HRTEM analysis of these clusters showed that the core size is about 1.2 nm which is in agreement with the size of clusters protected by individual proteins. A four-fold enhancement in the emission intensity of ∼Au36@BSA–Lyz was observed, when compared to that of the individual clusters when excited at the same wavelength. The quantum yield of ∼Au36@BSA–Lyz was found to be 42.4% which is extremely high compared to that of the already reported clusters. Proximity of two different proteins around a core can enhance FRET and thus, the emission intensity. The presence of multiple cluster species in solution or cluster protected by mixed protein systems might be another reason for such a high quantum yield. Understanding the system in detail is limited by several factors. Separation of the specific entity from the mixture of free proteins and other clusters can help to understand the system in greater detail. Similarly studies such as small angle X-ray scattering and spectroscopy may help to understand the system in more detail, which is part of our future investigations. Such a system can be used for sensing ultralow levels of analytes, because of the sensitivity of protein protected clusters to a range of analytes. Being bio-compatible, these can be used as fluorescent tags and can replace fluorescent dyes for staining biological entities and tracking biomolecules in real systems.Acknowledgements
AB, JSM and TP thank the Department of Science and Technology, Government of India for continuous support of their research program on nanomaterials. AB thanks the Council of Scientific and Industrial Research (CSIR) for his fellowship. JSM thanks IIT Madras for a fellowship. TP and HL thank the India-Korea research program for a joint project.References
- R. Jin, Nanoscale, 2010, 2, 343–362 RSC.
- A. Mathew and T. Pradeep, Part. Part. Syst. Charact., 2014, 31, 1017–1053 CrossRef CAS PubMed.
- T. Udayabhaskararao and T. Pradeep, J. Phys. Chem. Lett., 2013, 4, 1553–1564 CrossRef CAS.
- P. L. Xavier, K. Chaudhari, A. Baksi and T. Pradeep, Nano Rev., 2012, 3, 14767 CAS and the references cited therein.
- D. M. Chevrier, A. Chatt and P. Zhang, J. Nanophotonics, 2012, 6, 064504 CrossRef PubMed.
- M. W. Heaven, A. Dass, P. S. White, K. M. Holt and R. W. Murray, J. Am. Chem. Soc., 2008, 130, 3754–3755 CrossRef CAS PubMed.
- M. Zhu, C. M. Aikens, F. J. Hollander, G. C. Schatz and R. Jin, J. Am. Chem. Soc., 2008, 130, 5883–5885 CrossRef CAS PubMed.
- D. Crasto, S. Malola, G. Brosofsky, A. Dass and H. Hakkinen, J. Am. Chem. Soc., 2014, 136, 5000–5005 CrossRef CAS PubMed.
- H. Qian, W. T. Eckenhoff, Y. Zhu, T. Pintauer and R. Jin, J. Am. Chem. Soc., 2010, 132, 8280–8281 CrossRef CAS PubMed.
- C. Zeng, H. Qian, T. Li, G. Li, N. L. Rosi, B. Yoon, R. N. Barnett, R. L. Whetten, U. Landman and R. Jin, Angew. Chem., Int. Ed., 2012, 51, 13114–13118 CrossRef CAS PubMed.
- P. D. Jadzinsky, G. Calero, C. J. Ackerson, D. A. Bushnell and R. D. Kornberg, Science, 2007, 318, 430–433 CrossRef CAS PubMed.
- H. Yang, Y. Wang, H. Huang, L. Gell, L. Lehtovaara, S. Malola, H. Häkkinen and N. Zheng, Nat. Commun., 2013, 4, 2422 Search PubMed.
- A. Desireddy, B. E. Conn, J. Guo, B. Yoon, R. N. Barnett, B. M. Monahan, K. Kirschbaum, W. P. Griffith, R. L. Whetten, U. Landman and T. P. Bigioni, Nature, 2013, 501, 399–402 CrossRef CAS PubMed.
- J. Hassinen, P. Pulkkinen, E. Kalenius, T. Pradeep, H. Tenhu, H. Hakkinen and R. H. A. Ras, J. Phys. Chem. Lett., 2014, 5, 585–589 CrossRef CAS.
- A. Mathew, G. Natarajan, L. Lehtovaara, H. Hakkinen, R. M. Kumar, V. Subramanian, A. Jaleel and T. Pradeep, ACS Nano, 2014, 8, 139–152 CrossRef CAS PubMed.
- Y. Niihori, M. Matsuzaki, T. Pradeep and Y. Negishi, J. Am. Chem. Soc., 2013, 135, 4946–4949 CrossRef CAS PubMed.
- T. Pradeep, A. Baksi and P. L. Xavier, in Functional Nanometer-Sized Clusters of Transition Metals: Synthesis, Properties and Applications, The Royal Society of Chemistry, 2014, pp. 169–225 Search PubMed.
- N. Goswami, K. Zheng and J. Xie, Nanoscale, 2014, 6, 13328–13347 RSC.
- J. T. Petty, J. Zheng, N. V. Hud and R. M. Dickson, J. Am. Chem. Soc., 2004, 126, 5207 CrossRef CAS PubMed.
- J. Zheng, C. Zhang and R. M. Dickson, Phys. Rev. Lett., 2004, 93, 077402 CrossRef.
- J. Xie, Y. Zheng and J. Y. Ying, J. Am. Chem. Soc., 2009, 131, 888–889 CrossRef CAS PubMed.
- L. Shang, Y. Wang, J. Jiang and S. Dong, Langmuir, 2007, 23, 2714–2721 CrossRef CAS PubMed.
- M. A. Habeeb Muhammed, P. K. Verma, S. K. Pal, A. Retnakumari, M. Koyakutty, S. Nair and T. Pradeep, Chem.–Eur. J., 2010, 16, 10103–10112 CrossRef CAS PubMed.
- Y. Liu, K. Ai, X. Cheng, L. Huo and L. Lu, Adv. Funct. Mater., 2010, 20, 951–956 CrossRef CAS PubMed.
- A. Mathew, P. R. Sajanlal and T. Pradeep, J. Mater. Chem., 2010, 21, 11205–11212 RSC.
- J. Xie, Y. Zheng and J. Y. Ying, Chem. Commun., 2010, 46, 961–963 RSC.
- J. S. Mohanty, P. L. Xavier, K. Chaudhari, M. S. Bootharaju, N. Goswami, S. K. Pal and T. Pradeep, Nanoscale, 2012, 4, 4255–4262 RSC.
- H.-W. Li, K. Ai and Y. Wu, Chem. Commun., 2011, 47, 9852–9854 RSC.
- U. Anand, S. Ghosh and S. Mukherjee, J. Phys. Chem. Lett., 2012, 3, 3605–3609 CrossRef CAS.
- P.-H. Chan and Y.-C. Chen, Anal. Chem., 2012, 84, 8952–8956 CAS.
- K. Chaudhari, P. L. Xavier and T. Pradeep, ACS Nano, 2011, 5, 8816–8827 CrossRef CAS PubMed.
- P. L. Xavier, K. Chaudhari, P. K. Verma, S. K. Pal and T. Pradeep, Nanoscale, 2010, 2, 2769–2776 RSC.
- H. Wei, Z. Wang, L. Yang, S. Tian, C. Hou and Y. Lu, Analyst, 2010, 135, 1406–1410 RSC.
- A. Baksi, P. L. Xavier, K. Chaudhari, N. Goswami, S. K. Pal and T. Pradeep, Nanoscale, 2013, 5, 2009–2016 RSC.
- A. Baksi, T. Pradeep, B. Yoon, C. Yannouleas and U. Landman, ChemPhysChem, 2013, 14, 1272–1282 CrossRef CAS PubMed and the references cited there in.
- A. Baksi and T. Pradeep, Nanoscale, 2013, 5, 12245–12254 RSC.
- C.-L. Liu, H.-T. Wu, Y.-H. Hsiao, C.-W. Lai, C.-W. Shih, Y.-K. Peng, K.-C. Tang, H.-W. Chang, Y.-C. Chien, J.-K. Hsiao, J.-T. Cheng and P.-T. Chou, Angew. Chem., Int. Ed., 2011, 50, 7056–7060 CrossRef CAS PubMed.
- Y. Yu, Z. Luo, C. S. Teo, Y. N. Tan and J. Xie, Chem. Commun., 2013, 49, 9740–9742 RSC.
- H.-Y. Liu, X. Zhang, X.-M. Wu, L.-P. Jiang, C. Burda and J.-J. Zhu, Chem. Commun., 2011, 47, 4237 RSC.
- Y. Yue, T.-Y. Liu, H.-W. Li, Z. Liu and Y. Wu, Nanoscale, 2012, 4, 2251–2254 RSC.
- L. Yan, Y. Cai, B. Zheng, H. Yuan, Y. Guo, D. Xiao and M. M. F. Choi, J. Mater. Chem., 2012, 22, 1000–1005 RSC.
- T.-H. Chen and W.-L. Tseng, Small, 2012, 8, 1912–1919 CrossRef CAS PubMed.
- G. Guan, S.-Y. Zhang, Y. Cai, S. Liu, M. S. Bharathi, M. Low, Y. Yu, J. Xie, Y. Zheng, Y.-W. Zhang and M.-Y. Han, Chem. Commun., 2014, 50, 5703–5705 RSC.
- S. D. Haveli, P. Walter, G. Patriarche, J. Ayache, J. Castaing, E. E. Van, G. Tsoucaris, P.-A. Wang and H. B. Kagan, Nano Lett., 2012, 12, 6212–6217 CrossRef CAS PubMed.
- M. Li, D.-P. Yang, X. Wang, J. Lu and D. Cui, Nanoscale Res. Lett., 2013, 8, 182 CrossRef PubMed.
- C. Shao, B. Yuan, H. Wang, Q. Zhou, Y. Li, Y. Guan and Z. Deng, J. Mater. Chem., 2011, 21, 2863 RSC.
- X. Wen, P. Yu, Y.-R. Toh, A.-C. Hsu, Y.-C. Lee and J. Tang, J. Phys. Chem. C, 2012, 116, 19032–19038 CAS.
- X. Xia, Y. Long and J. Wang, Anal. Chim. Acta, 2013, 772, 81–86 CrossRef CAS PubMed.
- M. A. H. Muhammed and T. Pradeep, in Advanced Fluorescence Reporters in Chemistry and Biology II, ed. A. P. Demchenko, Springer Berlin Heidelberg, 2010, pp. 333–353 Search PubMed.
- S. Wang, X. Meng, A. Das, T. Li, Y. Song, T. Cao, X. Zhu, M. Zhu and R. Jin, Angew. Chem., Int. Ed., 2014, 53, 2376–2380 CrossRef CAS PubMed.
- Z. Luo, X. Yuan, Y. Yu, Q. Zhang, D. T. Leong, J. Y. Lee and J. Xie, J. Am. Chem. Soc., 2012, 134, 16662–16670 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Comparative MALDI-MS of BSA and ∼Au30@BSA at higher and lower mass range, MALDI-MS of Lyz and Au+–Lyz, BSA–Lyz adducts, XPS spectrum, UV-Vis spectra, concentration dependent MALDI-MS of AgQC@BSA–Lyz. See DOI: 10.1039/c5ra06964e |
| ‡ Contributed equally. |
| This journal is © The Royal Society of Chemistry 2015 |