Prediction of novel crystal structures and superconductivity of compressed HBr†
Abstract
The crystal structures of HBr under high pressure have been explored systematically using the particle swarm optimization method. Two new stable structures (I![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 2d and C2/m) were predicted above 100 GPa which have lower energies than the previously suggested P
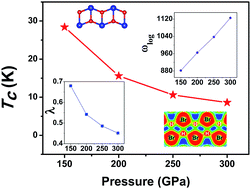
2d and C2/m) were predicted above 100 GPa which have lower energies than the previously suggested P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) structure. A sequence of phase transitions from molecular to cluster, to chain, and then to the atomic phase was revealed. Enthalpy and phonon calculations affirm the thermodynamic stability of the high-pressure polymorphs. Significantly, both high pressure phases (C2/m and Pmmn) were found to be superconducting with a maximum critical temperature close to 30 K at 150 GPa. The present results provide a clear high-pressure transformation pathway that helps to understand the structural evolution and superconductivity of highly compressed HBr.
structure. A sequence of phase transitions from molecular to cluster, to chain, and then to the atomic phase was revealed. Enthalpy and phonon calculations affirm the thermodynamic stability of the high-pressure polymorphs. Significantly, both high pressure phases (C2/m and Pmmn) were found to be superconducting with a maximum critical temperature close to 30 K at 150 GPa. The present results provide a clear high-pressure transformation pathway that helps to understand the structural evolution and superconductivity of highly compressed HBr.

 Please wait while we load your content...
Please wait while we load your content...