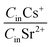Open Access Article
Open Access ArticlePore structure and sorption characterization of titanosilicates obtained from concentrated precursors by the sol–gel method
O.
Oleksiienko
*ab,
S.
Meleshevych
a,
V.
Strelko
a,
Ch.
Wolkersdorfer
bc,
M. M.
Tsyba
a,
Yu. M.
Kylivnyk
a,
I.
Levchuk
b,
M.
Sitarz
d and
M.
Sillanpää
b
aInstitute of Sorption and Problems of Endoecology NAS of Ukraine, 13 General Naumov str., Kyiv 03164, Ukraine. E-mail: ovalexis@gmail.com; Tel: +380 970870818
bLaboratory of Green Chemistry, Lappeenranta University of Technology, Sammonkatu 12, 50130 Mikkeli, Finland
cTshwane University of Technology, Faculty of Science, Department of Environmental, Water and Earth Science, 175 Nelson Mandela Drive, Pretoria 0001, South Africa
dAGH University of Science and Technology, Faculty of Materials Science and Ceramics, Al. Mickiewicza 30, 30-059 Kraków, Poland
First published on 26th August 2015
Abstract
A modified approach using the sol–gel synthesis of titanosilicate (TiSi) with a developed pore structure under relatively mild synthesis conditions was developed. To identify the controlling factors for the preparation of gels with the desired properties, the influences of the synthesis parameters on the structure and sorption capacity of the materials were studied. The chosen synthesis conditions allowed the preparation of TiSi xerogels with a high decontamination factor for both Sr2+ and Cs+. Effects of competitive ions on the separation factors, distribution and selectivity coefficients were studied from different background solutions containing Sr2+ and Cs+ simultaneously. It could be shown that it is possible to produce TiSi gels with developed pore structures that are capable of removing Sr2+ and Cs+ from complex aqueous solutions.
Introduction
Titanosilicates (TiSi) are inorganic compounds widely applied in sorption and ion-exchange processes.1–11 These chemically and thermally stable and radiation resistant materials with a high ion-exchange rate and remarkable capacity, are able to effectively decontaminate liquid radioactive wastes (LRW) from long-live nuclides, such as 90Sr, 134,137Cs, 233–235,238U, 241Am, 239,240Pu and 154Eu.1,3,12,13 In numerous works, different ways of TiSi synthesis, such as hydrolysis of alkoxides and inorganic salts via hydrothermal, microwave, mechanochemical and sol–gel routes were proposed.14–29 Using alkoxides as precursors leads to high purity materials. However, for the synthesis with alkoxides the following drawbacks were observed: toxic, expensive and unstable precursors, ecotoxic solvents and poor reproducibility of results. Consequently, inorganic precursors become now more commonly used for the synthesis due to their stability, easy handling and lower costs.1,8,15 As a ligand, hydrogen peroxide (H2O2) is traditionally used for slowing down the hydrolysis rate of Ti-containing precursors to the silicon precursor rate and increasing the amount of co-precipitated Ti–O–Si bonds. Advantages of using such a ligand are its eco-friendliness and non toxicity of the products H2O and O2. The main disadvantage is that the released oxygen during the hydrothermal treatment (HTT) raises the autogenous pressure inside the autoclave to more than 70 bar. Due to this fact, special equipment is required. In preceding works, microwave and mechanochemical routes as well as other reported methods yielded formation of powder titanosilicates.16–29 However, it is well known that many effective powder materials are not suitable for wide practical applications due to agglomeration, which decreases the working surface and active centre accessibility, difficulties with handling and filtration (stoppages, high hydrostatic resistance, bulk compression and poor wettability).30,31Sol–gel synthesis has numerous benefits in comparison to other methods, such as an excellent homogenization of the precursors during the sol preparation stage, a possibility to obtain high purity materials with the desired composition, structure and properties under comparably mild conditions and an opportunity to choose the form of materials (powders, fibers, films or particles).32 Synthesis for titanosilicate preparation via the sol–gel method using H2O2 was based on the approach reported in ref. 33. Previously, decomposition of the peroxide ligand released the oxygen that ruined the formed gel and the titanosilicates could be obtained only in powder form.
Recently, the first authors of this paper developed and patented a new sol–gel synthesis of titanosilicates from inorganic precursors based on gel preparation at room temperature,34,35 which overcomes several of the before mentioned disadvantages. This synthesis method leads to stable materials with reproducible properties. The crucial point for the proposed approach is the possibility to use chemicals with essential concentrations (2–5 M) strongly increasing the material yield. This synthesis route can be accomplished using a range of eco-friendly compounds, such as polyalcohols and organic hydroxy-, ceto-, di- and tricarboxylic acids and its mixtures. Utilization of such ligands solved a few problems: at first, the proposed ligands did not ruin the gel structure as in the traditional H2O2 method, and the TiSi xerogels obtained had particle sizes of 4 mm and less, instead of being a fine powder. The second advantage is the decrease of the autogenous pressure in the autoclaves by at least an order of magnitude. Finally, substituting the H2O2 ligand offers an opportunity to increase the precursor number and decrease the resulting material costs. The possibility to replace the TiCl4 precursor with pure and technical TiOSO4 solution was shown earlier.34–37 In addition, the pure silica sources Na2SiO3 and K2SiO3 have been replaced with cheaper bulk liquid glasses and stable porous gels with a high sorption ability to Sr2+ were obtained after such precursor substitutions. Using inexpensive precursors (technical TiOSO4, containing iron cations) would not be possible with H2O2, due to the catalytic effect of Fe3+ on the hydrogen peroxide decomposition. The modified approach presented in this paper, thus, is very promising for the large-scale application of TiSi materials.
The first part of this paper describes and exemplifies the newly developed approach in the synthesis of TiSi xerogels from pure titanyl sulphate, sodium liquid glass and sodium hydroxide. In the second part, the effect of the synthesis parameters on structural and sorption characteristics is evaluated in order to obtain a sorption material highly efficient for Sr nuclides. The third part is devoted to assessing the ability to uptake stable Cs+ and Sr2+ nuclides from aqueous solutions containing both potential pollutants simultaneously.
Experimental session
Chemicals
Titanium tetrachloride (TiCl4), metatitanic acid (H2TiO3) and technical solutions of liquid glass (Na2O·2.5SiO2) were used as precursors (Sumykhimprom and Kremnypolymer, Ukraine). A bulk solution of Na2O·2.5SiO2 was taken for synthesis after filtering through a polypropylene filter (0.5–1.0 mm). D-Sorbitol ((2S,3R,4R,5R)-hexane-1,2,3,4,5,6-hexol (C6H14O6)), L-lactic acid (2-hydroxypropanoic acid (C3H6O3)), benzene (C6H6), Trilon B (disodium ethylenediaminetetraacetic acid (EDTA)) or (2-({2-[bis(carboxymethyl)amino]ethyl}(carboxymethyl)amino)acetic acid) and calcein (fluorexon) indicator (2-[[7′-[[bis(carboxymethyl)amino]methyl]-3′,6′-dihydroxy-3-oxospiro[2-benzofuran-1,9′-xanthene]-2′-yl]methyl-(carboxymethyl)amino]acetic acid) with an assay of 95–98% (Chimlaborreaktiv, Macrochem and Synbias, Ukraine) were used without further purification. Pure solutions of TiOSO4 were achieved by replacing Cl− with SO42− in TiCl4 or by dissolving the metatitanic acid in H2SO4. All chemicals used for the sorption tests (CsCl, SrCl2·6H2O; NaCl, NaHCO3, KCl, CaCl2, D-glucose, H2SO4, NaOH) were of analytical grade (Sigma-Aldrich) and used without further purification. Laboratory glassware was rinsed with concentrated HCl or HNO3, and Milli-Q water (resistance 18.2 MΩ) was used for all studies.Synthesis
Titanosilicate sols were obtained by mixing two initial solutions at room temperature using a magnetic stirrer. The first solution was prepared by mixing pure (3.4 M) TiOSO4 and the ligand, a mixture with D-sorbitol and L-lactic acid. The second initial solution was obtained by mixing a technical liquid glass solution (3.81 M of Si) with 5 M NaOH. Gels for the present study were achieved by mixing the precursor at the molar ratio of Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Si
Si![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ligand
ligand![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NaOH = 1
NaOH = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5
0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. The obtained colourless transparent sols were transformed into milky homogeneous gels after 7 s to 15 min (depending on the pH). Fresh gels were aged at room temperature under a polyethylene cover for 0–7 d (days) in order to investigate the effects of aging time (Tag) before hydrothermal treatment (HTT) on the gel properties. Syneresis liquids were taken for pH measurements and gels were then subject to HTT in Teflon-lined steel autoclaves under autogenous pressure. Influences of the HTT parameters were studied in the temperature range of 120–200 °C during 6–48 h.34,35 The proposed synthesis can be described as follows:
4. The obtained colourless transparent sols were transformed into milky homogeneous gels after 7 s to 15 min (depending on the pH). Fresh gels were aged at room temperature under a polyethylene cover for 0–7 d (days) in order to investigate the effects of aging time (Tag) before hydrothermal treatment (HTT) on the gel properties. Syneresis liquids were taken for pH measurements and gels were then subject to HTT in Teflon-lined steel autoclaves under autogenous pressure. Influences of the HTT parameters were studied in the temperature range of 120–200 °C during 6–48 h.34,35 The proposed synthesis can be described as follows: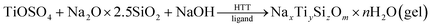 | (1) |
The HTT gels were rinsed with water to remove salts and ligands. Thereafter, the hydrogels were dried at 80 °C, ground, sieved and fractionated.
Characterization
Sample phase identification was done using X-ray diffraction (XRD), patterns collected using DRON-4-07 (CuKα; 2θ = 5–80° step Δ2θ = 0.02°). The Brunauer–Emmett–Teller's (BET) specific surface area and pore structure were determined using a Quantachrome NOVA 2200 surface area analyser and calculated with the NOVAWin software. The pore size distribution, micropore and total pore volume of TiSi xerogels were evaluated by density functional theory (DFT) and Dubinin–Radushkevich (DR) equations.38,39 The following express-test methods were used: exsiccator porosimetry and trilonometric titration of Sr2+. TiSi xerogels were dried at 105 °C until constant weight was achieved and thereafter saturated with distilled water and benzene vapours in an exsiccator to constant weight at room temperature. The maximum specific sorption pore volume Vp, which is relevant for the characterization and sorption capacity of the studied material, was calculated with eqn (2) (cm3 g−1). A single point measurement of maximum sorption capacity q to Sr2+ from 0.05 M SrCl2 solution was used as an express-test of the sorption ability (eqn (3)). Experimental conditions of the sorption test were: room temperature (25 ± 2 °C), contact time 24 h, 0.1 M NaCl background solution. The concentration of Sr2+ in solution was estimated by trilonometric titration with a calcein (fluorexon) indicator and calculated with eqn (4):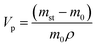 | (2) |
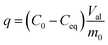 | (3) |
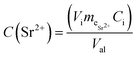 | (4) |
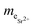 is the mass equivalent of Sr2+ (meq L−1); C0 and Ceq are the initial and equilibrium concentrations of Sr2+ in solution (mg L−1, mmol L−1 or meq L−1).
is the mass equivalent of Sr2+ (meq L−1); C0 and Ceq are the initial and equilibrium concentrations of Sr2+ in solution (mg L−1, mmol L−1 or meq L−1).
The sorption ability in regards to Sr2+ and Cs+ present simultaneously in aqueous solutions was evaluated by batch experiments. Initial Sr2+ concentrations were varied from 4.45 × 10−4 to 1.26 mmol L−1, while the initial Cs+ concentration was kept constant at 0.752 mmol L−1. Furthermore, initial Cs+ concentrations varied from 6.85 × 10−6 to 0.75 mmol L−1 with a constant initial Sr2+ concentration of 1.26 mmol L−1. Milli-Q water, 0.1 M NaCl and Ringer–Locke's solution (0.15 mol L−1 of NaCl, 2.38 mmol L−1 of NaHCO3, 2.68 mmol L−1 of KCl, 0.2 g L−1 of CaCl2 and 1.8 mmol L−1 of D-glucose; pH = 8.07 (ref. 40 and 41)) were used as media for the sorption tests. An IKA KS 4000i control shaker-incubator was used at 200 rpm (rotations per minute) to overcome the slow diffusion rate; the aliquot volume to sorbent material mass ratio (V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) m) was 100; contact time 24 h; ambient temperature. The Cs+ and Sr2+ concentrations during the sorption experiments were determined by inductively coupled plasma with mass detector (ICP-MS) model Agilent 7500ce. Distribution coefficients (Kd) and decontamination factors (DF) were calculated using the following equations:
m) was 100; contact time 24 h; ambient temperature. The Cs+ and Sr2+ concentrations during the sorption experiments were determined by inductively coupled plasma with mass detector (ICP-MS) model Agilent 7500ce. Distribution coefficients (Kd) and decontamination factors (DF) were calculated using the following equations:
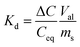 | (5) |
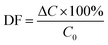 | (6) |
| ΔC = C0 − Ceq | (7) |
Separation factor F and selectivity coefficients were calculated using the following equations:42–44
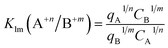 | (8) |
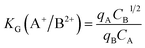 | (9) |
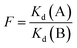 | (10) |
Results and discussion
To identify the crucial synthesis parameters for creating materials with the anticipated properties, the fresh homogenous TiSi hydrogels were subjected to various post synthesis treatments. Altering parameters such as pH or pressure of the HTT yielded material with poor sorption characteristics of Sr2+ as well as decomposed gel structures resulting in powder like materials. Drying the fresh hydrogels before HTT produced materials with DF(Sr2+) ≤ 80%. Therefore, these synthesis factors were excluded from the present study. Yet, variations of the aging time Tag before HTT, the duration or temperature of HTT resulted in TiSi materials with a high affinity to Sr2+. In order to understand the influence of the abovementioned factors on the TiSi gels' structure, studies using the low temperature nitrogen sorption/desorption technique and X-ray diffraction were conducted subsequently.Fig. 1 and Table 1 illustrate the influence of aging time (Tag) before HTT on the TiSi gel structural and sorption properties. Pore structure parameters measured with the surface area analyser were compared with data obtained by express-analysis methods (sorption pore volume by water and benzene vapours and sorption capacity to Sr2+). The results presented are for xerogels HTT under 150 °C during 6 h.
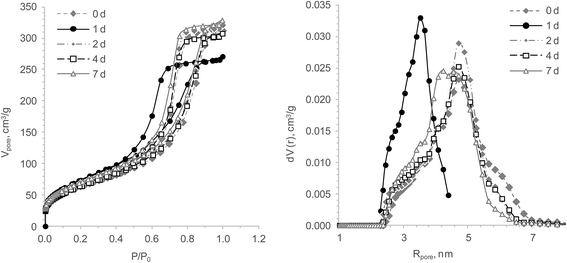 | ||
| Fig. 1 Effects of the aging time before HTT on the TiSi xerogels' structural and sorption properties: isotherms of nitrogen sorption/desorption (left) and DFT pore size distribution (right). | ||
| No. | T ag, d | HTT | S BET a, m2 g−1 | V pore total a, cm3 g−1 | V DR micro a, cm3 g−1 | R pore a, nm | V w b, cm3 g−1 | V b b, cm3 g−1 | q(Sr2+)b, mmol g−1 | |
|---|---|---|---|---|---|---|---|---|---|---|
| t, °С | τ, h | |||||||||
| a The instrumental error was 1–3%. b The experimental error was up to 10%. | ||||||||||
| 1 | 0 | 150 | 6 | 229.2 | 0.48 | 0.09 | 4.71 | 0.34 | 0.48 | 0.86 |
| 2 | 1 | 150 | 6 | 270.3 | 0.41 | 0.10 | 3.52 | 0.31 | 0.40 | 0.91 |
| 3 | 2 | 150 | 6 | 244.1 | 0.50 | 0.06 | 4.71 | 0.37 | 0.45 | 0.98 |
| 4 | 4 | 150 | 6 | 233.6 | 0.48 | 0.06 | 4.71 | 0.33 | 0.41 | 1.04 |
| 5 | 7 | 150 | 6 | 257.0 | 0.51 | 0.07 | 4.39 | 0.31 | 0.40 | 0.85 |
| p | — | — | — | 0.715 | 0.140 | 0.254 | 0.012 | 0.384 | 0.159 | 0.511 |
It was found that the isotherms obtained from TiSi gels belong to the IV type. According to the IUPAC classification45 such an isotherm form is typical for materials with a developed pore structure. The shape of the hysteresis loops can be attributed to the H2 type that suggested the “ink-bottle” pore form.
A statistical test with SPSS (22.0) investigated if there are statistically significant differences in the TiSi gel properties or sorption capacities of the HT treatments with different aging times. The Shapiro–Wilk test for normality showed that for 7 of the 8 parameters the zero-hypothesis for normality can't be rejected and that the aging time has no measurable effect on the TiSi properties. Yet, TiSi no. 2, relating to one day of aging, has a Rpore value that is different from the other 4 aging steps. Based on the statistical investigation that sample is an outlier and the difference is very likely due to random processes during the HTT.
Sorption tests with Sr2+ proved that the reported synthesis approach led to TiSi gels with a high sorption capacity. The sorption data for Sr2+ shows a gradual increase of the TiSi sorption capacity (q) of 18% during the first four days and decreases thereafter. Yet, based on the statistical investigation it cannot be excluded that this observation is due to random behaviour. Since the statistical investigation shows that the sorption capacity does not depend on the aging period Tag, a one day aging period was chosen for all further investigations to simplify sample handling.
Effects of the HTT duration were studied at 150 °C using hydrogels aged one day. It became clear that the studied variation has an essential effect on the pore structure and is reflected in the sorption pore volume evaluated by benzene vapour (Fig. 2 and Table 2). Between 6 and 24 h HTT, the total pore volume and pore radius increased, while the specific surface area decreased. This observation indicates that the HTT conditions catalyse the ripening processes while the syneresis cannot compensate it since water vapour has less adhesive properties than liquid water at normal conditions. As a result, the mean pore radius rose while the micropore volume and sorption capability to Sr2+ did not change. These suggestions are based on express-test data and were confirmed with low temperature nitrogen studies.
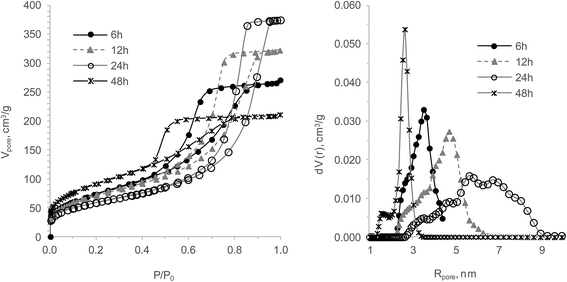 | ||
| Fig. 2 The effect of the HTT duration on the TiSi xerogels' structural and sorption properties: isotherms of nitrogen sorption/desorption (left) and DFT pore size distribution (right). | ||
| No. | T ag, d | HTT | S BET, m2 g−1 | V pore total, cm3 g−1 | V DR micro, cm3 g−1 | R pore, nm | V w, cm3 g−1 | V b, cm3 g−1 | q(Sr2+), mmol g−1 | |
|---|---|---|---|---|---|---|---|---|---|---|
| t, °С | τ, h | |||||||||
| 2 | 1 | 150 | 6 | 270.3 | 0.42 | 0.10 | 3.52 | 0.31 | 0.40 | 0.91 |
| 6 | 1 | 150 | 12 | 256.2 | 0.50 | 0.07 | 4.81 | 0.31 | 0.47 | 0.93 |
| 7 | 1 | 150 | 24 | 209.4 | 0.58 | 0.08 | 5.64 | 0.31 | 0.94 | 0.89 |
| 8 | 1 | 150 | 48 | 326.0 | 0.33 | 0.08 | 2.64 | 0.32 | 0.31 | 0.96 |
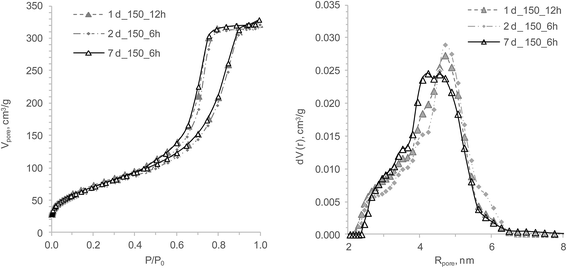
The data shows that an increase of the HTT duration from 6 to 12 h is similar to 7 d of aging before HTT, as the samples for 1, 2 and 7 aging days with 6 and 12 h show almost identical isotherms (Fig. 3). The pore size distributions of these gels illustrate stiffness (less sensitivity to HTT influence) of the hydrogel structure after one week of aging before HTT. The sample with the highest specific surface area and the widest hysteresis loop had been prepared during 48 h of HTT (Fig. 2). The size and shape of the hysteresis loop indicates an essential difference between the pores “necks” and “bodies”. The pore size distribution curve of the sample with 48 h HTT confirms a biporous structure. The biggest shift of the isotherm in a region of lower relative pressure is an indication for a structure with small mean pore sizes. Consequently, a hydrogel with an analogous structure would be obtained after a few months of aging. Interestingly, the maximal Sr2+ sorption capacity varies around 7% within the limits of the experimental error. It can therefore be concluded that the effect of both studied factors on the micropore volume and developed transport (mesopore) structure of all obtained gels is insignificant. This observation agrees with earlier works11,14,33,46–48 stating that the ion-exchange of small cations like Cs+ and Sr2+ takes place in micropores and crystal channels with micropore size. Consequently, it was decided to use 6 h of HTT for further studies as the least energy consuming synthesis of the effective sorption materials investigated. Because of the small number of observations (4), a statistical test with Shapiro–Wilk for normality did not provide useful results.
Influences of the HTT temperature on the hydrogels' structure aged one day are presented in Fig. 4 and Table 3. It could be shown that HTT at 120 °C promoted the formation of a TiSi hydrogel structure with an atypical isotherm attributed to the V type. This type and the “open” shape of the hysteresis loop are indicative of a microporous structure of the sample and consequently a small pore volume. Using pore distribution data, the micropore sample structure was evaluated. The sorption pore volume by water vapour and the sorption capacity to Sr2+ of this xerogel are half as large as those of sample no. 2 at 150 °С. The HTT at 150–200 °С results in hydrogels with a type IV isotherm. It should be noted that a hydrogel with an H1 hysteresis loop shape was formed at 175 °С HTT. This fact testifies the presence of a macropore or mesopore structure with a big pore radius. The pore size distribution of this sample is wide and the mean pore radius is about 9 nm. Samples with 150 and 200 °C have an H2 shape of the hysteresis loop (Fig. 4).
| No. | T ag, d | HTT | S BET, m2 g−1 | V pore total, cm3 g−1 | V DR micro, cm3 g−1 | R pore, nm | V w, cm3 g−1 | V b, cm3 g−1 | q(Sr2+), mmol g−1 | |
|---|---|---|---|---|---|---|---|---|---|---|
| t, °С | τ, h | |||||||||
| 9 | 1 | 120 | 6 | 186.9 | 0.007 | 0.0001 | 1.77 | 0.12 | 0.05 | 0.49 |
| 2 | 1 | 150 | 6 | 270.3 | 0.419 | 0.101 | 3.52 | 0.31 | 0.40 | 0.91 |
| 10 | 1 | 175 | 6 | 176.2 | 0.70 | 0.05 | 8.96 | 0.26 | 0.64 | 0.83 |
| 11 | 1 | 200 | 6 | 236.3 | 0.44 | n.d. | 4.10 | 0.30 | 0.42 | 0.47 |
| 12 | 7 | 200 | 6 | 292.7 | 0.53 | 0.07 | 4.07 | 0.28 | 0.46 | 0.65 |
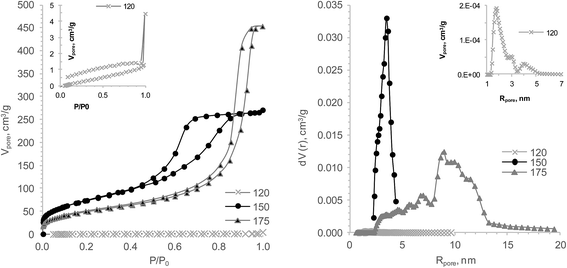 | ||
| Fig. 4 The influence of the HTT temperature on the TiSi xerogels' properties: isotherms of nitrogen sorption/desorption (left) and DFT pore size distribution (right). | ||
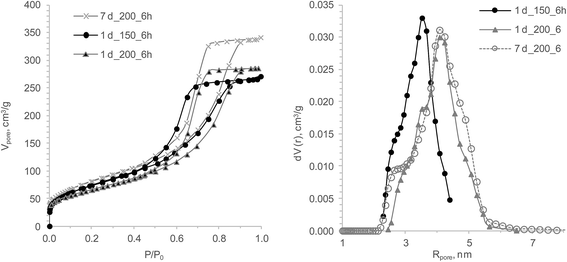
The influence of temperature on the xerogels' pore structure differs substantially from that of varying HTT durations and aging times (Fig. 5). Temperature clearly predetermined the pore structure and, as a result, the xerogels' sorption capacity. It was found that rising the HTT temperature from 120 °C to 150 °С almost doubled q(Sr2+) while in the temperature interval from 175 to 200 °С, q(Sr2+) decreased by 76%. This can be explained with the fact that the 200 °C samples have only half of the micropore volume. Consequently, 175 °C is the temperature where polycondensation, hydrolysis and coarsening processes have the highest rate and/or the basic pH of the syneresis liquid has a catalytic effect on the abovementioned processes. Because of the different modifications in the treatment (aging and temperature), a statistical test with the data did not provide additional information (Table 3).
 | ||
| Fig. 5 The influence of the HTT temperature and the aging before HTT on the TiSi xerogels' properties: isotherms of nitrogen sorption/desorption (left) and DFT pore size distribution (right). | ||
Unexpectedly low q(Sr2+) were obtained for the samples treated at 200 °C HTT and after one and seven days of aging prior to HTT. The shape of the isotherms, hysteresis loops and pore distributions suggests that the developed porosity with micropore volume should be enough for a high sorption ability. Yet, the decline of the sorption capacity might be ascribed to changes in the surface chemistry of the hydrogels under higher temperatures or a decreasing accessibility of the ion-exchange centres due to an increasing order of the structure.
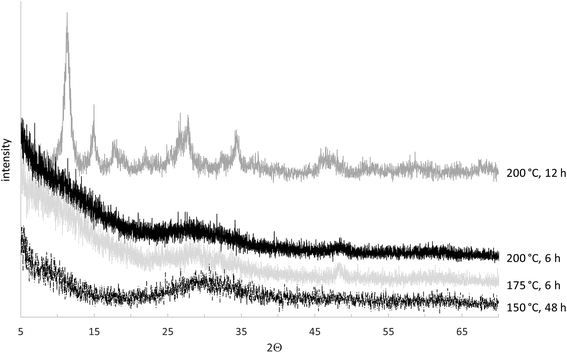
For justification of this assumption, an XRD investigation was performed. The effect of the temperature on structural changes is reflected in the XRD patterns (Fig. 6). The spectrum peaks have the same position and broadness as poorly crystalline sodium titanosilicate.49 Consequently, the described synthetic approach lead to a TiSi which would have the ideal formula Na2Ti2O3(SiO4)·2H2O were it a highly crystalline sodium titanosilicate. According to the results, crystallites become detectable after HTT at 200 °C and the molar ratio of the Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Si would be an important parameter in the sorption study. Yet, in this study, even after 48 h of HTT at 150 °C, no detectable crystalline phase was found and the molar ratio is of no known relevance. Thus, it can be concluded that the temperature had the strongest effect on the TiSi hydrogel structure. It was further assumed that the duration of the HTT could be used instead of pre-HTT aging to reduce the synthesis time for the final product. Detailed structural and sorption studies should be done around 175 °C HTT in order to obtain sorption material for big cation pollutants. Following the synthesis conditions: Tag ≥ 1 day and HTT at 150 °C for 6 h make it possible to obtain materials with the highest sorption capacity in regards to Sr2+, consuming a minimum of time and energy. Samples prepared under the chosen regime were synthesized for further sorption investigations.
Si would be an important parameter in the sorption study. Yet, in this study, even after 48 h of HTT at 150 °C, no detectable crystalline phase was found and the molar ratio is of no known relevance. Thus, it can be concluded that the temperature had the strongest effect on the TiSi hydrogel structure. It was further assumed that the duration of the HTT could be used instead of pre-HTT aging to reduce the synthesis time for the final product. Detailed structural and sorption studies should be done around 175 °C HTT in order to obtain sorption material for big cation pollutants. Following the synthesis conditions: Tag ≥ 1 day and HTT at 150 °C for 6 h make it possible to obtain materials with the highest sorption capacity in regards to Sr2+, consuming a minimum of time and energy. Samples prepared under the chosen regime were synthesized for further sorption investigations.
Sorption study
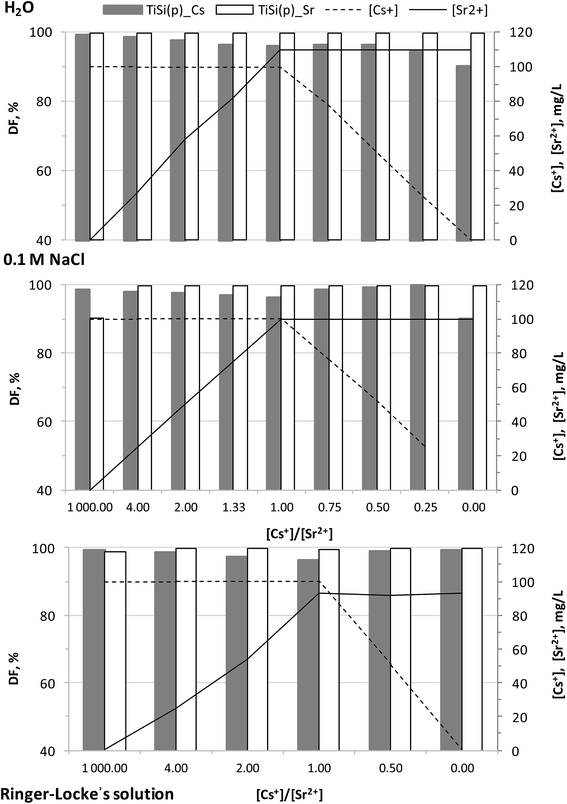
The effects of the synthesis conditions on the studied materials' sorption capacity in relation to Sr2+ were evaluated from 0.1 M NaCl by trilonometric titration. For determining the sorption capability and selectivity of the obtained xerogels, sorption tests were performed from solutions containing both Sr2+ and Cs+ simultaneously. The 0.1 M NaCl background solution was used to assess the Na+ competition effect on the potential pollutants' uptake. A Ringer–Locke's solution was used to evaluate the effect of Na+, K+ and Ca2+ competitive ions on the sorption capacity and the selectivity of the synthesized xerogels. There were two reasons to expect a higher affinity and selectivity to Sr2+ compared to the cations Na+, K+ and Ca2+. Firstly, it is well known that in general, cations with a higher valence more preferably sorb compared to those with lower ones. When the valence is equal, the cation with a bigger atomic mass is sorbed.41,42 Secondly, studies of TiSi xerogel sorption capacities in the same background solutions with only one cation (Sr2+ or Cs+) indicate different sorption mechanisms for these cations (chemisorption for Sr2+ and activated sorption for Cs+).36,37 Coefficients of selectivity and separation factors were calculated in order to evaluate these assumptions. The collected data is expected to be useful for predicting the TiSi behaviour in aqueous media containing Sr2+, Cs+, Na+, K+ and Ca2+ such as drinking, ground, sea and mine water, blood plasma and liquid radioactive wastes.Titanosilicates synthesized via the sol–gel method have a high sorption affinity to both Sr2+ and Cs+ (Fig. 7). Varying the initial concentration of one potential pollutant cation has a weak effect on the decontamination factor DF of both cations. Generally, the synthesized material has a higher affinity and sorption capacity to Sr2+: q(Sr2+) > q(Cs+) and Kd(Sr2+) > Kd(Cs+). It is worth to note, that the same values of q(Sr2+) and q(Cs+) were obtained during sorption studies in the same background solutions with only one cation (Sr2+ or Cs+). It could be shown that the separation factor has a lesser deviation from experimental observations than the calculated selectivity coefficients (Table 4). The selectivity to Sr2+ in water increased with increasing initial Sr and decreasing Cs concentrations. The presence of competitive ions in the background solutions reduced both, the selectivity coefficients and separation factors. For example, the Gapon's selectivity coefficient KG for the mass concentration ratio of Sr2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cs+ = 1 in media with dissolved salts was only half of that one in water. Nevertheless, the synthesized TiSi xerogels demonstrated a high sorption ability, DF and selectivity to Cs+ even in the presence of competing cations such as Na+, K+, Sr2+ and Ca2+. These good characteristics could be observed even from trace initial concentrations of the potential pollutant (C0 ≤ 10−3 mmol L−1).
Cs+ = 1 in media with dissolved salts was only half of that one in water. Nevertheless, the synthesized TiSi xerogels demonstrated a high sorption ability, DF and selectivity to Cs+ even in the presence of competing cations such as Na+, K+, Sr2+ and Ca2+. These good characteristics could be observed even from trace initial concentrations of the potential pollutant (C0 ≤ 10−3 mmol L−1).
| a | q(Cs+), mmol g−1 | K d(Cs+) | Cs+/Sr2+ | q(Sr2+), mmol g−1 | K d(Sr2+) | Sr2+/Cs+ | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| K lm | K G | F | K lm | K G | F | |||||
| a Mass concentration ratio. | ||||||||||
| H 2 O | ||||||||||
| 103 | 0.07 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 417 417 |
1.89 | 30.23 | 0.29 | 4.45 × 10−5 | 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 294 294 |
0.53 | 0.03 | 3.49 |
| 4 | 0.07 | 6167 | 1.06 | 0.65 | 0.18 | 0.03 | 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 744 744 |
0.94 | 1.55 | 5.47 |
| 2 | 0.07 | 4251 | 0.66 | 0.27 | 0.10 | 0.07 | 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 362 362 |
1.51 | 3.64 | 9.73 |
| 1.33 | 0.07 | 2667 | 0.37 | 0.13 | 0.05 | 0.09 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 997 997 |
2.67 | 7.64 | 19.10 |
| 1 | 0.07 | 2330 | 0.32 | 0.10 | 0.04 | 0.12 | 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 073 073 |
3.10 | 10.24 | 22.35 |
| 0.75 | 0.06 | 2686 | 0.39 | 0.12 | 0.06 | 0.13 | 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 726 726 |
2.57 | 8.52 | 17.77 |
| 0.5 | 0.04 | 2589 | 0.38 | 0.12 | 0.06 | 0.13 | 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 816 816 |
2.61 | 8.67 | 17.70 |
| 0.25 | 0.02 | 1687 | 0.27 | 0.08 | 0.04 | 0.13 | 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 217 217 |
3.71 | 12.30 | 23.25 |
| 10−4 | 6.18 × 10−7 | 912 | 0.16 | 0.05 | 0.03 | 0.13 | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 292 292 |
6.23 | 20.64 | 35.40 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||||
| 0.1 N NaCl | ||||||||||
| 103 | 0.07 | 6148 | 6.44 | 711.50 | 6.75 | 9.35 × 10−7 | 911 | 0.16 | 0.001 | 0.15 |
| 4 | 0.07 | 4434 | 0.66 | 0.42 | 0.10 | 0.03 | 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 237 237 |
1.52 | 2.390 | 10.20 |
| 2 | 0.07 | 3745 | 0.67 | 0.30 | 0.12 | 0.06 | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 138 138 |
1.49 | 3.330 | 8.32 |
| 1.33 | 0.07 | 3123 | 0.65 | 0.24 | 0.13 | 0.09 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 314 314 |
1.55 | 4.220 | 7.47 |
| 1 | 0.07 | 2677 | 0.59 | 0.19 | 0.13 | 0.11 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 725 725 |
1.70 | 5.360 | 7.74 |
| 0.75 | 0.06 | 6206 | 0.97 | 0.31 | 0.15 | 0.11 | 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 385 385 |
1.04 | 3.270 | 6.67 |
| 0.5 | 0.04 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 739 739 |
2.47 | 0.78 | 0.41 | 0.11 | 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 572 572 |
0.40 | 1.280 | 2.41 |
| 0.25 | 0.02 | 68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 999 999 |
12.59 | 4.00 | 2.30 | 0.11 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 053 053 |
0.08 | 0.250 | 0.44 |
| 10−4 | 6.17 × 10−7 | 911 | 0.18 | 0.06 | 0.03 | 0.11 | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 932 932 |
5.69 | 17.980 | 29.55 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||||
| Ringer–Locke's solution | ||||||||||
| 103 | 0.07 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 241 241 |
4.59 | 36.50 | 1.59 | 1.81 × 10−4 | 8326 | 0.22 | 0.03 | 0.63 |
| 4 | 0.07 | 6554 | 0.96 | 0.61 | 0.14 | 0.03 | 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 976 976 |
1.05 | 1.65 | 7.17 |
| 2 | 0.07 | 3728 | 0.72 | 0.31 | 0.14 | 0.06 | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 766 766 |
1.39 | 3.21 | 7.18 |
| 1 | 0.07 | 2595 | 0.57 | 0.19 | 0.13 | 0.11 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 505 505 |
1.75 | 5.30 | 7.90 |
| 0.5 | 0.04 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 314 314 |
1.59 | 0.52 | 0.24 | 0.11 | 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 198 198 |
0.63 | 1.92 | 4.09 |
| 10−3 | 1.19 × 10−4 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 642 642 |
2.78 | 0.91 | 0.44 | 0.11 | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 254 254 |
0.36 | 1.09 | 2.28 |
Conclusions
In the presented study, a synthetic approach yielding in materials with a poorly crystalline sodium titanosilicate structure at relatively mild conditions is described. The work showed that the temperature of the HTT has the strongest influence on the structure of the materials and consequently, it can be used as the controlling factor for the preparation of gels with the desired properties. The chosen synthesis conditions allow for the preparation of TiSi with a high decontamination factor for both Sr2+ and Cs+, and competitive ions have a weak influence on the ability and sorption capacity of the obtained TiSi xerogels. Based on the results, it could be shown that the presence of competitive ions reduced the selectivity coefficients and separation factors, but TiSi illustrates a higher affinity and selectivity to Sr2+ than to Cs+ in all studied media and concentrations. Hence, it can be concluded that the prepared materials could be used as an effective sorption material for Sr2+ and Cs+ decontamination from drinking, ground, sea and mine waters, blood plasma and LRW. Further studies need to show that the material can also be used in a pilot scale or industrial scale application.Acknowledgements
The authors thank Elisa Alasuvanto for her assistance with the sorption experiments. Also, we thank N. M. Patryliak, for assistance with the single point measurement of maximum sorption capacity to Sr2+. We are also grateful to Valerij I. Yakovlev for assisting with the precursor preparation and for our fruitful discussions. Furthermore, we thank Mykola P. Ryzhuk for supporting the hydrothermal experiment. Comments from two anonymous reviewers helped to emphasize the novelties described in this paper.References
- K. Popa and C. C. Pavel, Desalination, 2012, 293, 78–86 CrossRef CAS.
- Y. D. Noh, S. Komarneni and K. J. D. Mackenzie, Sep. Purif. Technol., 2012, 95, 222–226 CrossRef CAS.
- T. Möller, R. Harjula and J. Lehto, Sep. Purif. Technol., 2002, 28, 13–23 CrossRef.
- L. Lv, M. P. Hor, F. Su and X. S. Zhao, J. Colloid Interface Sci., 2005, 287, 178–184 CrossRef CAS PubMed.
- C. B. Lopes, M. Otero, Z. Lin, C. M. Silva, E. Pereira, J. Rocha and A. C. Duarte, J. Hazard. Mater., 2010, 175, 439–444 CrossRef CAS PubMed.
- S. Cavenati, C. A. Grande, F. V. S. Lopes and A. E. Rodrigues, Microporous Mesoporous Mater., 2009, 121, 114–120 CrossRef CAS.
- C. B. Lopes, M. Otero, Z. Lin, C. M. Silva, J. Rocha, E. Pereira and A. C. Duarte, Chem. Eng. J., 2009, 151, 247–254 CrossRef CAS.
- A. Clearfield, Solvent Extr. Ion Exch., 2000, 18, 655–678 CrossRef CAS.
- A. Clearfield, L. N. Bortun and A. I. Bortun, React. Funct. Polym., 2000, 43, 85–95 CrossRef CAS.
- J. H. Choi, S. D. Kim, Y. J. Kwon and W. J. Kim, Microporous Mesoporous Mater., 2006, 96, 157–167 CrossRef CAS.
- A. Anson, C. C. H. Lin, S. M. Kuznicki and J. A. Sawada, Chem. Eng. Sci., 2009, 64, 3683–3687 CrossRef CAS.
- P. Sylvester, E. A. Behrens, G. M. Granziano and A. Clearfield, Sep. Sci. Technol., 1999, 34, 1981–1992 CrossRef CAS.
- G. Lujaniene, S. Meleshevych, V. Kanibolotskyy, J. Sapolaite, V. Strelko, V. Remeikis, O. Oleksiienko, K. Ribokaite and T. Sciglo, J. Radioanal. Nucl. Chem., 2009, 282, 787–791 CrossRef CAS.
- A. Clearfield, Solvent Extr. Ion Exch., 2000, 18, 655–678 CrossRef CAS.
- J. Rocha and M. W. Anderson, Eur. J. Inorg. Chem., 2000, 5, 801–818 CrossRef.
- S. E. B. Garcia, K. Yamamoto and A. Muramatsu, J. Mater. Sci., 2008, 43, 2367–2371 CrossRef CAS.
- K. Yamamoto, S. E. B. Garcia, F. Saito and A. Muramatsu, Chem. Lett., 2006, 35, 570–571 CrossRef CAS.
- D. Coutinho, J. A. Losilla, J. Balkus and J. Kenneth, Microporous Mesoporous Mater., 2006, 90, 229–236 CrossRef CAS.
- X. Deng, Y. Wang, L. Shen, H. Wu, Y. Liu and M. He, Ind. Eng. Chem. Res., 2013, 52, 1190–1196 CrossRef CAS.
- T. Fernandez, U. K. Samersen, X. Joseph and N. V. Unnikrishnan, J. Mater. Process. Technol., 2008, 202, 528–535 CrossRef CAS.
- F. Figueras and J. L. Flores Moreno, in Emerging Fields in Sol–Gel Science and Technology, ed. T. Lopez, D. Avnir and M. Aegerter, Springer, US, 2003, pp. 37–48 Search PubMed.
- S. E. B. García, K. Yamamoto, F. Saito and A. Muramatsu, J. Jpn. Pet. Inst., 2007, 50, 53–60 CrossRef.
- T. Iwasaki, M. Isaka, H. Nakamura, M. Yasuda and S. Watano, Microporous Mesoporous Mater., 2012, 150, 1–6 CrossRef CAS.
- L. Liu, W. Tan, P. Xiao and Y. Zhai, Int. J. Miner., Metall. Mater., 2012, 19, 675–678 CrossRef CAS.
- M. Muthuraman and K. C. Patil, Mater. Res. Bull., 1998, 33, 655–661 CrossRef CAS.
- Z. Shan, E. Gianotti, J. C. Jansen, J. A. Peters, L. Marchese and T. Maschmeyer, Chem.–Eur. J., 2001, 7, 1437–1443 CrossRef CAS.
- S. Sivakumar, C. P. Sibu, P. Mukundan, P. K. Pillai and K. G. K. Warrier, Mater. Lett., 2004, 58, 2664–2669 CrossRef CAS.
- K. Yamamoto, S. E. Borjas García and A. Muramatsu, Microporous Mesoporous Mater., 2007, 101, 90–96 CrossRef CAS.
- K. Yamamoto, S. E. B. Garcia, F. Saito and A. Muramatsu, Chem. Lett., 2006, 35, 570–571 CrossRef CAS.
- J. Bridgwater, Powder Technol., 1976, 15, 215–236 CrossRef.
- R. Freeman, Powder Technol., 2007, 174, 25–33 CrossRef CAS.
- C. J. Brinker and G. W. Scherer, Sol–gel science: the physics and chemistry of sol–gel processing, Academic Press, United States, 1990 Search PubMed.
- O. V. Oleksiienko, S. I. Meleshevych, V. V. Strelko, O. I. V'yunov, O. K. Matkovsky, V. G. Milgrandt, M. M. Tsyba and V. A. Kanibolotsky, Prob. Chem. Chem. Technol., 2013, 2, 101–105 Search PubMed.
- V. G. Kalenchuk, S. V. Meleshevych, V. A. Kanibolotsky, V. V. Strelko, O. V. Oleksiienko and N. M. Patryliak, A method for producing titanosilicate ion exchangers, Patent UA48457U, 2010.
- V. V. Strelko, S. V. Meleshevych, V. A. Kanibolotsky and O. V. Oleksiienko, A method for producing titanosilicate ion exchangers, Patent UA48457U, 2012.
- O. Oleksiienko, I. Levchuk, M. Sitarz, S. Meleshevych, V. Strelko and M. Sillanpää, J. Colloid Interface Sci., 2015, 438, 159–168 CrossRef CAS PubMed.
- O. Oleksiienko, I. Levchuk, M. Sitarz, S. Meleshevych, V. Strelko and M. Sillanpää, Desalin. Water Treat., 2015 DOI:10.1080/19443994.2014.1003103.
- A. Dada, A. Olalekan, A. Olatunya and O. Dada, J. Appl. Chem., 2012, 3, 38–45 Search PubMed.
- A. Dąbrowski, Adv. Colloid Interface Sci., 2001, 93, 135–224 CrossRef.
- W. R. Amberson, J. Flexner, F. R. Steggerda, A. G. Mulder, M. J. Tendler, D. S. Pankratz and E. P. Laug, J. Cell. Comp. Physiol., 1934, 5, 359–382 CrossRef CAS.
- J. P. Steel, Br. Med. J., 1927, 2, 1177–1178 CrossRef CAS PubMed.
- A. I. Bortun, L. N. Bortun and A. Clearfield, Solvent Extr. Ion Exch., 1997, 5, 909–929 CrossRef.
- I. Shainberg, N. I. Alperovitch and R. Keren, Clays Clay Miner., 1987, 35, 68–73 CAS.
- V. P. Evangelou and F. J. Coale, Soil Sci. Soc. Am. J., 1987, 51, 69–72 Search PubMed.
- K. S. W. Sing, Pure Appl. Chem., 1982, 54(11), 2201–2218 CrossRef.
- A. Clearfield, Solid State Sci., 2001, 3, 103–112 CrossRef CAS.
- E. A. Behrens, D. M. Poojary and A. Clearfield, Chem. Mater., 1996, 8, 1236–1244 CrossRef CAS.
- A. Tripathi, D. G. Medvedev and A. Clearfield, J. Solid State Chem., 2005, 178, 253–261 CrossRef CAS.
- A. Clearfield, D. G. Medvedev, S. Kerlegon, T. Bosser, J. D. Burns and J. Milton, Solvent Extr. Ion Exch., 2012, 30, 229–243 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |