Electrocatalytic properties of iron chalcogenides as low-cost counter electrode materials for dye-sensitized solar cells†
Jiahao Guo‡
ab,
Suxia Liang‡a,
Yantao Shi*a,
Bo Lia,
Ce Haoa,
Xuchun Wangb and
Tingli Ma*cd
aState Key Laboratory of Fine Chemicals, School of Chemistry, Dalian University of Technology, Dalian, 116024, P. R. China. E-mail: shiyantao@dlut.edu.cn
bCollege of Chemistry and Materials Engineering, Anhui Science and Technology University, Fengyang, Anhui 233100, P. R. China
cSchool Petroleum and Chemical Engineering, Dalian University of Technology, Panjin Campus, Panjin 124221, P. R. China. E-mail: tinglima@dlut.edu.cn
dGraduate School of Life Science and Systems Engineering Kyushu Institute of Technology, Kitakyushu, Fukuoka 808-0196, Japan
First published on 29th July 2015
Abstract
Developing cost-effective and highly electrocatalytic Pt-free counter electrode (CE) materials for triiodide reduction has become a major interest for dye-sensitized solar cells (DSCs). In a heterogeneous catalytic system, iron chalcogenides like FeS2 and FeSe2 have demonstrated excellent catalytic activity when serving as CE materials in DSCs. However, theoretical and experimental studies have yet to be conducted to investigate the catalytic activity of iron chalcogenides in energy conversion and storage devices under the same conditions. In this work, FeS2, FeSe2, and FeTe2 were selected as our research object to systematically investigate and compare the regulatory mechanisms of the changes in the catalytic activity of iron chalcogenides. Theoretical research reveals that the iodine adsorption and charge exchange of these three materials occurred efficiently in heterogeneous catalytic systems. Experimental results further show that these three materials exhibited excellent catalytic activities. The conversion efficiencies of the corresponding DSCs are comparable to those of the sputtered Pt CE. This study also provides a method to rationally screen cost-effective and highly efficient catalytic materials for electrocatalysis applications.
1. Introduction
Increasing energy demands have prompted researchers to exploit highly efficient, cost-effective, and environmentally friendly alternative energy conversion and storage devices.1 As a type of photovoltaic device that directly converts sunlight into electrical energy, dye-sensitized solar cells (DSCs) can satisfy these requirements; as such, DSCs have been intensively investigated.2–4 Typical DSCs are composed of a dye-sensitized mesoporous oxide layer (TiO2), an electrolyte containing an iodide/triiodide (I3−/I−) redox couple, and a counter electrode (CE) that regenerates reduced species in electrolytes.5 Fig. 1 shows the working principle of DSCs.A superior CE material generally exhibits high electrocatalytic activity, good electrical conductivity, and chemical stability to collect photogenerated electrons from an external circuit and reduce I3− into I− for subsequent dye regeneration.6 Therefore, Pt-based CEs prepared by either sputtering or pyrolysis have been widely used to fabricate DSCs. However, Pt-based CEs are usually characterized by various limiting factors including high cost, element scarcity, and decreased activity derived from serious corrosion cause by iodine species from an electrolyte solution; these factors likely impede the scalable applications of DSCs.7 Hence, cost-effective and highly electrocatalytic alternative CE materials should be developed.8–22
Iron (Fe) widely exists in the Earth's crust and ranks fourth among the most abundant elements, following oxygen, silicon, and aluminum. Fe compounds with various compositions, valence states, and crystal structures23,24 are abundant, cost effective, and environmentally friendly; furthermore, Fe compounds have been extensively investigated because of important optical, electrical, optoelectronic, and transport properties.25–31 Some Fe compounds that exhibit excellent catalytic activity and stability can also serve as electrocatalysts (for oxygen reduction reaction) in fuel cells and other kinds of electrochemical cells.32,33 Other Fe compounds are efficient electrocatalysts as CEs for DSCs. For example, Wang et al.34 reported that DSCs with FeS2 nanocrystal-based CE exhibit a power conversion efficiency (PCE) of 7.31%. Yang et al.35 confirmed theoretically and experimentally that α-Fe2O3 nanocrystal is an excellent CE candidate with high electrocatalytic activity for I3− reduction. Our group36 further reported a composite catalyst (biomass carbon and Fe3O4) as a CE material for DSCs; using this composite catalyst, we obtained a high PCE of 8.11% in DSCs. We simultaneously introduced hierarchical Fe3O4 structures to DSCs as a CE with a PCE of 7.65%.37 In a heterogeneous catalytic system, iron chalcogenides have demonstrated excellent catalytic activity. As cathode catalysts, CE materials exhibit a catalytic activity greatly influenced by the outer electronic structure and surface morphological characteristics of electrode materials in a heterogeneous catalytic reaction.34
This study is the first to systematically illustrate the high catalytic activity of iron chalcogenides by using theoretical and experimental methods. We selected three iron chalcogenides FeS2, FeSe2, and FeTe2 as our research object from the outer electron of a nonmetallic element changes periodically. We initially calculated the iodine adsorption and charge exchange of the three iron chalcogenides on the basis of the density functional theory (DFT) theory; we found that these chalcogenides exhibit excellent catalytic activities in I3− reduction. We then synthesized FeS2 and FeSe2 by using a similar reductant-free solvothermal method. We also synthesized FeTe2 by using a solvent thermal method. Our experimental results further showed that these three samples exhibited excellent catalytic activities as CE materials involved in I3− reduction. DSCs based on these three CE materials also exhibited photoelectric properties comparable to DSCs based on Pt CE. Our research confirmed that iron chalcogenides are good electrocatalytic cathode materials. Therefore, these materials can be used to enhance the applications of iron chalcogenides.
2. Computational method
DFT computations were performed using projector augmented wave (PAW) potentials and Perdew–Burke–Ernerhof (PBE) functional implemented in Vienna ab initio simulation package (VASP).38–41 Relaxations were carried out using a conjugate-gradient algorithm. The selected energy convergence was 1 × 10−5 eV per atom, and relaxations were terminated if all of the forces were <0.01 eV Å−1. The cutoff energy was set as 364 eV for FeS2 and 347 eV for FeSe2 and FeTe2. The Brillouin zone was integrated using Monkhorst–Pack-generated sets of k points. We applied 15 × 15 × 15, 11 × 11 × 11, and 9 × 9 × 9 k-point meshes for FeS2, FeSe2, and FeTe2 in bulk computations, respectively. The occupancy of one-electron states was calculated using Gaussian smearing (SIGMA = 0.1 eV). The primitive cell of the three iron chalcogenides was used when the band structure was calculated.Surface computations were performed using a slab model. A typical low index surface (111) was considered in this study. FeS2 (111), FeSe2 (111), and FeTe2 (111) surfaces were modeled as a p (1 × 1) periodic slab; the vacuum between slabs was 20 Å. The atoms in the two bottom layers were fixed, and all other atoms were fully relaxed. A corresponding 3 × 3 × 1 k-point mesh was applied during optimization.
3. Experimental
3.1 Material preparation
3.2 Preparation of chalcogenides CEs and cell fabrication
FeS2, FeSe2, and FeTe2 CEs were prepared. In brief, 200 mg of FeS2, FeSe2, or FeTe2 powder and 4 g of ZrO2 pearl were dispersed in 4 ml isopropanol and milled for 4 h. The prepared chalcogenide slurry was sprayed onto an FTO glass (Asahi Glass, type-U, 14 U sq−1, Japan) with an airbrush (TD-128, Tiandi Co., Ltd.) connected to a mini compressor. The FTO substrate coated with an FeS2, FeSe2, or FeTe2 film was then annealed under a N2 atmosphere at 500 °C for 30 min in a tube furnace to obtain the expected FeS2, FeSe2, or FeTe2 CEs. The Pt CE was prepared in according with our previous work.42A TiO2 film (P25, Degussa, Germany) photoanode was fabricated via a screen-printing technique on the FTO conductive glass in accordance with previously described methods.43 The TiO2 film was immersed in a 5 × 10−4 M acetonitrile/tert-butyl alcohol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio) solution of cis-bis (isothiocyanato) bis(2,2ʹ-bipyridyl-4−4ʹ-dicarboxylato)-ruthenium(II) bis-tetrabutylammonium dye (N719, Solaronix SA, Switzerland; Fig. 2) at room temperature for 21 h to absorb the dye. The TiO2 photoanodes were then removed from the solution, rinsed with ethanol to remove the excess adsorbed dye, and dried in air at room temperature. A DSCs device was fabricated by placing the chalcogenide CEs on the N719 dye-sensitized TiO2 photoelectrode and clipped as an open cell for measurements. The DSCs device was then filled with the redox electrolyte composed of 0.06 M LiI, 0.6 M 1-butyl-3-methylimidazolium iodide, 0.03 M I2, 0.5 M 4-tert-butyl pyridine, and 0.1 M guanidiniumthiocyanate in acetonitrile solution via capillary force. The as-assembled DSCs with an active area of 0.16 cm2 were subjected to photovoltaic performance tests. A symmetrical cell was assembled by sandwiching two identical CEs with the same electrolyte as used in the DSCs assembly. The as-assembled symmetrical cell was then subjected to electrochemical impedance spectroscopy and Tafel polarization measurement.
1 volume ratio) solution of cis-bis (isothiocyanato) bis(2,2ʹ-bipyridyl-4−4ʹ-dicarboxylato)-ruthenium(II) bis-tetrabutylammonium dye (N719, Solaronix SA, Switzerland; Fig. 2) at room temperature for 21 h to absorb the dye. The TiO2 photoanodes were then removed from the solution, rinsed with ethanol to remove the excess adsorbed dye, and dried in air at room temperature. A DSCs device was fabricated by placing the chalcogenide CEs on the N719 dye-sensitized TiO2 photoelectrode and clipped as an open cell for measurements. The DSCs device was then filled with the redox electrolyte composed of 0.06 M LiI, 0.6 M 1-butyl-3-methylimidazolium iodide, 0.03 M I2, 0.5 M 4-tert-butyl pyridine, and 0.1 M guanidiniumthiocyanate in acetonitrile solution via capillary force. The as-assembled DSCs with an active area of 0.16 cm2 were subjected to photovoltaic performance tests. A symmetrical cell was assembled by sandwiching two identical CEs with the same electrolyte as used in the DSCs assembly. The as-assembled symmetrical cell was then subjected to electrochemical impedance spectroscopy and Tafel polarization measurement.
3.3 Characterizations of CE materials and DSCs
The X-ray powder diffraction (XRD) profiles of the as prepared samples were measured using an X-ray powder diffractometer (D/Max 2400, Rigaku, Japan) with Cu Kα radiation (λ = 0.154 nm). The surface morphological characteristics and microstructure of the chalcogenide samples were examined through field emission scanning electron microscopy (FEI HITACHI S-4800). Cyclic voltammetry (CV) was performed using a CHI630D electrochemical workstation (Chenhua, Shanghai) in a three-electrode system of an anhydrous acetonitrile solution containing 0.1 M LiClO4, 10 mM LiI, and 1 mM I2 at a scan rate of 10 mV s−1. The as-prepared chalcogenide CEs was assigned as a working electrode; a Pt wire was utilized as a counter electrode; an Ag/AgCl electrode was used as a reference electrode. Electrochemical impedance spectroscopy (EIS) measurements were conducted using the symmetrical cell on a computer-controlled electrochemical workstation (Zennium Zahner, Germany) under dark conditions. The geometric active area of the symmetrical cell was 0.64 cm2. The samples were scanned from 0.1 Hz to 1 MHz with a bias of −0.75 V; the AC amplitude was 10 mV. The obtained EIS data were analyzed using commercially available Z-view software and fitted in terms of an appropriate equivalent electric circuit. Tafel polarization experiments were conducted in an electrochemical workstation (CHI630D, Chenhua, Shanghai) with the symmetrical cell at a scan rate of 10 mV s−1. The photovoltaic performances of the DSCs were evaluated by a digital source meter (Keithley 2601, computer-controlled, USA) under simulated sunlight illumination condition, which was produced by a solar simulator (PEC-L15, Peccell, Japan). The light power density was calibrated against a Si-based reference cell to accurately simulate the full-sun intensity (100 mW cm−2).4. Results and discussion
4.1 Theoretical investigation
The DFT computations were performed using PAW potentials and PBE functional implemented in VASP to investigate the electrocatalytic activity of the three iron chalcogenides. We obtained a crystal lattice with parameter values through the full relaxation of atomic positions and lattice constants (Table S1†). Our theoretical results are consistent with the experimental values. The electrical conductivity of the CE materials is a critical factor that influences the catalytic activity of CEs in DSCs. To analyze the electronic properties, we determined the band structures of the three iron chalcogenides. The theoretical band gaps of FeS2, FeSe2, and FeTe2 are 0.55, 0.37, and 0.19 eV, respectively, which are consistent with those of Takashi.44 The results show that these chalcogenides are semiconductors with a small band gap and enhances electrical conductivity, which are essential for CE materials.On the interface of the CE/electrolyte, the main process is the reduction of electron acceptors from the electrolyte; this process can be expressed as follows: I3−(sol) + 2e− → 3I−(sol); the general consensus of the reaction mechanism can be described as follows:35
| I3−(sol) ↔ I2(sol) + I−(sol) | (1) |
| I2(sol) + 2* → 2I* | (2) |
| I* + e− → I−(sol) | (3) |
 | (4) |
| FeS2 (111) | FeSe2 (111) | FeTe2 (111) | |
|---|---|---|---|
| E0/ev | 3.59 | 1.89 | 2.54 |
| Ef/ev | −2.13 | −2.91 | −2.14 |
| Φ/ev | 5.72 | 4.80 | 4.68 |
| EIad/eV | 1.89 | 1.54 | 1.04 |
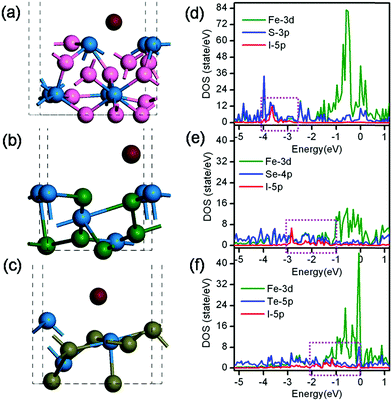
where E(interface), E(I2), and E(I/interface) are the energies of the interface, I2, and I adsorbed on the interface in the gas phase, respectively. Each individual energy term on the right side can be achieved directly through the DFT calculation. Fig. 3 shows the optimized structure of FeS2 (111), FeSe2 (111), and FeTe2 (111) surfaces with an I atom and the corresponding projected density of states (PDOS). The calculated adsorption energies of I on FeS2 (111), FeSe2 (111), and FeTe2 (111) surfaces were 1.89, 1.54, and 1.04 eV, respectively (Table 1). On the basis of the PDOS diagram, we found a very strong hybridization between iodine p orbital, iron d orbital, and chalcogen p orbital in the contour plot of the corresponding bonding state. The PDOS images suggest that I 5p, Fe 3d, and S 3p states hybridize well between −4 and −2.5 eV. This finding indicates that an orbital overlap occurs between I 5p, Fe 3d, and S 3p electrons; furthermore, this overlap is accounted for the relatively large binding energy of I atom adsorbed on the FeS2 (111) surfaces. On FeSe2 (111) surfaces, I 5p orbital interacts with Fe 3d and Se 4p orbitals between −3 and −1 eV. On the FeTe2 (111) surfaces, the orbital overlap region of I 5p, Fe 3d, and Te 5p states ranges from −2 eV to 0 eV. The locations of the orbital overlap of I with Fe, S, Se, and Te move gradually to Fermi level. This result may be accounted for the decreased adsorption of the I atom on FeS2 (111), FeSe2 (111), and FeTe2 (111) surfaces and for the increased interfacial charge transfer ability.
According to electronic catalysis theory on semiconductors, the catalytic activity of semiconductors is associated with conductivity and work function.46 To further understand interfacial charge transfer properties, we considered work function in our calculations. Work function (Φ) is equal to vacuum level (E0) minus Fermi level (Ef). The calculated work function of the FeS2, FeSe2, and FeTe2 (111) surfaces were 5.72, 4.80, and 4.68 eV, respectively. Based on the calculated results, our conclusion is that FeS2 yields a higher adsorption energy of I, and FeTe2 exhibits a lower work function; therefore, the FeS2 (111) surfaces are more beneficial for I2 dissociation, and the FeTe2 CE shows a more efficient electron transfer ability than the other CEs. Considering work function and adsorption energy, we found that three iron chalcogenides exhibit excellent catalytic activities for I3− reduction.
4.2 Morphological and structural characterizations of the materials
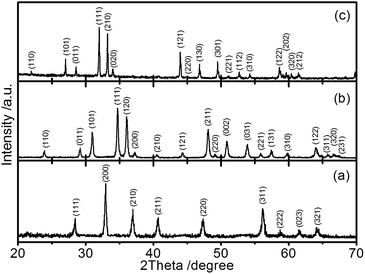
The crystallinity of CE materials affects the performance of DSCs. The crystallinity and structural data of the obtained iron chalcogenides were collected through XRD. Our results showed that the peaks of FeS2 can be indexed to diffractions from (111), (200), (210), (211), (220), (311), (222), (023), and (231) planes (Fig. 4). These findings are consistent with those of a standard FeS2 pyrite (PDF # 42-1340); the characteristic peaks of other impurities were not found. The results also indicate that the obtained FeS2 exhibits a well-crystallized pyrite structure. For FeSe2, the diffraction peaks at 24.04, 29.28, 31.04, 34.72, 36.22, 37.38, 40.68, 44.40, 48.19, 49.24, 50.92, 53.90, 55.91, 57.48, 59.91, 64.09, 65.86, 66.68, and 67.53 can be assigned to (110), (011), (101), (111), (120), (200), (210), (121), (211), (220), (002), (031), (221), (131), (310), (122), (311), (320), and (231) crystal planes, respectively. These diffraction peaks match well with a typical orthorhombic FeSe2 (PDF # 65-2570). In this study, FeTe2 was prepared with a solvothermal method; in this method, ethylenediamine, FeCl2, and Te powder were used as a solvent, an iron source, and a tellurium source, respectively. The products collected in different stages were examined through XRD (Fig. S1†). Using this approach, we found that the FeTe2 formation is relatively slow because the characteristic peaks of Te in the XRD patterns completely disappeared after 96 h. All of the diffraction peaks of the product collected at 96 h are consistent with those of a typical orthorhombic FeTe2 (PDF # 51-1158); the characteristic peaks of other impurities, such as Te were not detected. The lattice parameters of the three iron chalcogenides are listed in Table S1;† these results are in agreement with those of previous studies.47,48The three iron chalcogenides were analyzed through scanning electron microscopy (Fig. 5). The result showed that the products of FeS2 are in the form of either dispersed nanosized particles or aggregates (Fig. 5a and d). The sizes of nanoparticles ranged from 60 nm to 350 nm. In contrast to FeS2, FeSe2 shows an irregular and hierarchical flower-like structure consisting of nanorods with a diameter and length of approximately 30 and 300 nm (Fig. 5b and e). The FeTe2 sample is characterized by irregular and micron-sized polyhedrons (Fig. 2c and f). The evolution of the morphological characteristic of FeTe2 is shown in Fig. S2† and the details have been listed in the ESI.† Large particles usually cause a decrease in catalytic active sites; large particles may also affect the catalytic activity of FeTe2.
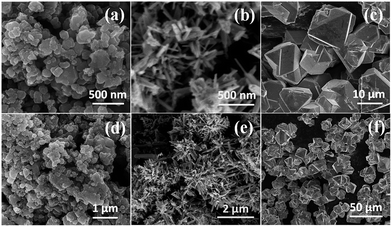 | ||
| Fig. 5 High- and low-magnification SEM images of FeS2 (a and d), FeSe2 (b and e), and FeTe2 (c and f). | ||
4.3 Electrocatalytic activity characterization of CEs
CV was performed in acetonitrile solution containing 10 mM LiI, 1 mM I2, and 0.1 M LiClO4 at a scanning rate of 10 mV s−1 to evaluate the electrochemical catalytic activities of the three chalcogenide CEs and Pt CEs for I3− reduction in an I−/I3− redox shuttle used in DSCs. Fig. 6 shows the CV curves of FeS2, FeSe2, FeTe2, and Pt-CE. Each electrode exhibits two typical pairs of oxidation/reduction peaks. The left pair in the CV plots corresponds to I3− + 2e− → 3I−; the right pair corresponds to 3I2 + 2e− → 2I3−. We focused on the characteristics of the left pair because the catalytic activity of a CE is related to the I3−/I− redox pair. On the basis of the reaction of I3−/I−, we found that the cathodic peak current density (Jpc1) and the anodic peak current density (Jpa1) correspond to I3− reduction and I− oxidation, respectively. The peak separation between anodic and cathodic peaks (Epp) is negatively correlated with the standard electrochemical rate constant of a redox reaction. Jpc1 and Epp are two critical parameters considered to compare electrocatalytic activities of different CEs. A higher reduction Jpc1 and a lower Epp indicate a higher catalytic activity.19 | ||
| Fig. 6 Cyclic voltammograms of the triiodide/iodide redox couple of the three chalcogenide CEs and Pt CEs. | ||
The CV profiles of the three chalcogenide CEs are similar to those of Pt-CE (Fig. 6); this result indicated that the three CEs exhibit abilities similar to Pt-CE in catalyzing the reduction of I3− to I−. Jpc1 of FeS2 and FeSe2 is slightly higher than that of Pt-CE; the cathodic peak potentials of the three CEs are also similar to those of Pt-CE; this finding indicated that I3− can be reduced more easily on FeS2 and FeSe2 CEs than on Pt-CE. By contrast, these two parameters are generally lower in FeTe2 CE than in Pt-CE; this finding corresponded to a slightly inferior catalytic activity. The relatively lower Jpc1 of FeTe2 can be attributed to insufficient surface area because of micron-sized particles. Epp of FeS2 and FeSe2 CEs is substantially similar to that of Pt (Table 2). Epp is also directly responsible for the comparable electrocatalytic activities of the three CEs; moreover, this parameter is accounted for the similar overpotential loss of FeS2, FeSe2, and Pt-CE in DSCs. High peak current densities and similar Epp of FeS2 and FeSe2 electrodes suggest that FeS2 and FeSe2 promote the high reversibility of I3−/I− redox reaction and remarkable electrocatalytic activity of I3− reduction; this characteristic is a prerequisite for an excellent CE material in DSCs. Furthermore, FeTe2 CE yields a high Epp (199 mV), which indicates that the electrocatalytic activity of FeTe2 CE is slightly inferior to that of FeS2 and FeSe2 electrodes. The electrochemical stability of a CE is of great importance in terms of additional DSC applications. We measured the sequential CVs for 120 cycles by using three iron chalcogenide electrodes, and the result is shown in Fig. S3.† A slight variation was observed in current density; therefore, these iron chalcogenide CEs exhibit good stability.
| CEs | Rs/Ωcm2 | Rct/Ωcm2 | CPE/μF | ZN/Ωcm2 | Epp/mV | J0/mA cm−2 | VOC/mV | JSC/mA cm−2 | FF | PCE/% |
|---|---|---|---|---|---|---|---|---|---|---|
| Pt | 8.27 | 0.64 | 42.2 | 0.45 | 143 | 5.82 | 747 | 15.28 | 0.67 | 7.70 |
| FeS2 | 8.91 | 0.44 | 96.1 | 2.35 | 138 | 6.31 | 753 | 15.55 | 0.68 | 8.00 |
| FeSe2 | 8.65 | 0.55 | 208.3 | 0.69 | 151 | 5.72 | 769 | 14.23 | 0.72 | 7.92 |
| FeTe2 | 7.51 | 1.08 | 61.9 | 9.92 | 199 | 4.21 | 716 | 15.34 | 0.66 | 7.21 |
EIS is a powerful electrochemical method to characterize intrinsic interfacial charge transfer and charge transport kinetics at an electrode/electrolyte interface. We conducted EIS measurements by using symmetrical FeS2/FeS2, FeSe2/FeSe2, FeTe2/FeTe2, and Pt/Pt electrochemical cells under dark conditions; we then investigated the charge transfer at the electrode/electrolyte interface. The obtained Nyquist plots of the four CEs are presented in Fig. 7; two semicircles can be observed at high- (left) and low-frequency (right) regions. The results were then fitted using Z-view software, and the equivalent circuit diagram shown in the inset in Fig. 7a. The relevant EIS parameters of the four symmetrical cells are summarized in Table 2. In general, a high-frequency intercept on the real axis represents series resistance (Rs), which is mainly composed of the bulk resistance of CE materials, the resistance of the FTO substrate, and the contact resistance, among others. The left semicircle at the high-frequency range corresponds to the charge transfer resistance (Rct) at the CE/electrolyte interface involved in I3− reduction; the corresponding constant phase angle element indicates the deviation from ideal capacitance because of electrode roughness. The right semicircle at the low-frequency range corresponds to Nernst diffusion impedance (ZN) of I3−/I− redox species transport in the electrolyte. In Table 2, Rs values of FeS2, FeSe2, FeTe2, and Pt CEs were 8.91, 8.65, 7.51, and 8.27 Ωcm2, respectively, which may be related to electrode conductivity.
FeTe2 CE yielded the smallest Rs, which may be attributed to a relatively higher electrical conductivity; this result is consistent with that of previous theoretical calculation. Rs can be improved by optimizing fabrication conditions to significantly enhance the photovoltaic performance of DSCs.49 Rct of symmetric cells based on FeS2, FeSe2, FeTe2, and Pt CEs are 0.44, 0.55, 1.08, and 0.64 Ωcm2, respectively. This finding indicated that the three chalcogenide CEs exhibited a high catalytic activity implicated in I3− reduction in DSCs and may also be attributed to high intrinsic catalytic activity. FeTe2 CE presented a higher ZN than Pt CE; this higher ZN may be the main factor causing a relatively low electrocatalytic activity.37 Furthermore, the higher ZN of FeTe2 may be caused by larger particles, resulting in a decrease in the number of catalytic active sites and a slightly inferior catalytic activity. Indeed, the conclusions derived from EIS and CV data are consistent. Moreover, FeS2 and FeSe2 CEs can compete with Pt CE in DSCs applications.
Tafel polarization tests were conducted to further elucidate the interfacial charge transfer properties of the I3−/I− redox shuttle on an electrode surface. Fig. 8 shows the Tafel polarization curves of the symmetrical cells based on FeS2, FeSe2, FeTe2, and Pt CEs. In theory, a Tafel curve is composed of three zones: polarization zone represented by a curve at low potentials (|V| < 120 mV); a Tafel zone represented by a curve at moderate potentials (with a sharp slope); and a diffusion zone represented by a curve at high potentials (horizontal part). In the Tafel zone, the intersection of a cathodic branch and an equilibrium potential line can be regarded as exchange current density (J0), which can be obtained by extending the line to zero voltage and by measuring the intercept on the y-axis. A steep gradient of the Tafel zone corresponds to a high J0 to some degree. The cathodic branches of the Tafel curves of FeS2 and FeSe2 CEs display larger slopes than those of Pt-CE and FeTe2 CE (Fig. 8); this result suggests that high J0 is generated from FeS2 and FeSe2 electrodes. This result also indicates that FeS2 and FeSe2 electrodes can trigger the reduction of I3− to I− as effectively as Pt CE. In theory, J0 changes inversely with Rct, which is consistent with EIS values, as expressed in eqn (5), where R is the gas constant, T is the temperature, F is Faraday's constant, n is the number of electrons exchanged in the reaction at the electrolyte/CE interface, and Rct is the charge transfer resistance.
 | (5) |
In the diffusion zone, the intersection of the cathodic branch with the y-axis shows the limiting diffusion current density (Jlim), which is determined on the basis of the diffusion properties of a redox couple and CE catalysts, as expressed in eqn (6), where D is the diffusion coefficient, l is the spacer thickness, and c is the I3− concentration. A high Jlim indicates a large diffusion coefficient and a small ZN at the same potential. FeS2 CE yields Jlim similar to Pt CE. By contrast, FeSe2 CE shows a slightly low Jlim; this result suggests a high diffusion velocity of I3− in the electrolyte, thereby causing a relatively higher photovoltaic performance when FeSe2 is used as CE. FeTe2 CE exhibited moderate Jlim values.
 | (6) |
Based on these theoretical and experimental results of the comprehensive analysis of the electrocatalytic activity of CEs for the I3−/I− redox couple, our conclusion is that the three iron chalcogenides showed excellent catalytic activity for the I3− reduction; these chalcogenides are very suitable as CE materials of DSCs. FeTe2 presents a slightly inferior catalytic activity possibly attributed to the larger particles than other CEs. Therefore, the catalytic activity of FeTe2 can be improved through the regulation of morphological characteristics.
4.4 Photovoltaic performance of DSCs
To demonstrate the catalytic activity of the three chalcogenide CEs characterized by theoretical and experimental methods, we assembled DSCs with FeS2, FeSe2, and FeTe2 CEs; we then investigated the photocurrent–voltage (J–V) curves (Fig. 9). Table 2 summarized the corresponding photovoltaic parameters. The DSCs based on FeS2 CE exhibited a short-circuit photocurrent (JSC) of 15.55 mA cm−2, an open-circuit voltage (VOC) of 753 mV, a fill factor (FF) of 0.68, and a power conversion efficiency (PCE) of 8.00%. The DSCs based onFeSe2 CE yielded JSC of 14.23 mA cm−2, VOC of 769 mV, FF of 0.72, and PCE of 7.92%. The DSCs based on sputtering Pt CE displayed JSC of 15.28 mA cm−2, VOC of 747 mV, FF of 0.67, and PCE of 7.70%. Therefore, FeS2- or FeSe2-based DSCs showed a comparable (even slightly higher) PCE to sputtering Pt-based DSCs; this finding can be mainly attributed to the slight improvement in JSC and FF of the DSCs with FeS2 CE and in VOC and FF of the DSCs with FeSe2. FF may be improved because of a relatively small impedance of chalcogenides CE; these finding are consistent with those of EIS experiments. The DSCs based on FeTe2 CE exhibited lower VOC and FF than those based on other CEs; PCE of 7.21% was obtained. The relatively higher VOC reflects the higher electrocatalytic activity of FeS2 and FeSe2 CEs, which shifted the redox potential of I3−/I− to the positive direction.50 FeS2 and FeTe2 showed relatively higher JSC, which could be derived from an excellent catalytic activity of FeS2 and good electrical conductivity for FeTe2. By contrast, FeSe2 yielded a relatively small JSC, which could be related to the nanorod structure. Indeed, FeTe2 CE shows a good electrocatalytic activity in I3− reduction. A slightly lower PCE of the FeTe2-based DSCs may be caused by the larger particles of this material; as a result, the number of catalytic active sites is decreased. Therefore, the catalytic activity of FeTe2 can be improved by adjusting morphological characteristics. | ||
| Fig. 9 Photocurrent–voltage (J–V) curves of DSCs based on the three chalcogenides and Pt CEs determined under simulated sunlight illumination conditions (Xe arc lamp, 1.5 AM, 100 mW cm−2). | ||
5. Conclusions
In summary, the electrolytic behaviors of FeS2, FeSe2, and FeTe2 on the reduction of I3− in DSCs were systematically investigated using theoretical and experimental methods. Combining the calculated results of adsorption energy and work function, we suggest that the three iron chalcogenides showed excellent catalytic activities for I3− reduction. A very strong hybridization among I p orbital, Fe d orbital and chalcogen p orbital indicated a strong interaction among these elements. Electrochemical experimental results demonstrated that pyrite FeS2 nanoparticles, orthorhombic FeSe2 nanorods, and orthorhombic FeTe2 micron bulks showed superior electrolytic activities. The DSCs with iron chalcogenides CEs exhibited photovoltaic performances comparable to the DSCs based on Pt CE. Our findings further indicated that iron chalcogenides displayed excellent electrocatalytic activities. This study also provided a method to rationally screen cost-effective and highly efficient catalytic materials for electrocatalysis applications.Acknowledgements
Financial support provided by the National Natural Science Foundation of China (Grant No. 51402036, 51273032 and 91333104), International Science & Technology Cooperation Program of China (Grant No. 2013DFA51000), the Fundamental Research Funds for the Central Universities (Grant No. DUT15YQ109), and Natural Science Foundation of Anhui Province (Grant No. KJ2013A079). This research was also supported by the State Key Laboratory of Fine Chemicals of China.Notes and references
- G. Q. Zhang, B. Y. Xia, X. Wang and X. W. (David) Lou, Adv. Mater., 2014, 26, 2408 CrossRef CAS PubMed.
- M. Grätzel, Nature, 2001, 414, 338 CrossRef PubMed.
- F. Gao, Y. Wang, D. Shi, J. Zhang, M. Wang, X. Jing, R. Humphry-Baker, P. Wang, S. M. Zakeeruddin and M. Grätzel, J. Am. Chem. Soc., 2008, 130, 10720 CrossRef CAS PubMed.
- A. Yella, C. L. Mai, S. M. Zakeeruddin, S. N. Chang, C. H. Hsieh, C. Y. Yeh and M. Grätzel, Angew. Chem., 2014, 126, 3017 CrossRef PubMed.
- B. O'Regan and M. Grätzel, Nature, 1991, 353, 737 CrossRef PubMed.
- N. Papageorgiou, Coord. Chem. Rev., 2004, 248, 1421 CrossRef CAS PubMed.
- M. Wang, A. M. Anghel, B. Marsan, N. C. Ha, N. Pootrakulchote, S. Zakeeruddin and M. Grätzel, J. Am. Chem. Soc., 2009, 131, 15976 CrossRef CAS PubMed.
- M. X. Wu, X. Lin, A. Hagfeldt and T. L. Ma, Angew. Chem., Int. Ed., 2011, 50, 3520 CrossRef CAS PubMed.
- S. N. Yun, M. X. Wu, Y. D. Wang, J. Shi, X. Lin, A. Hagfeldt and T. L. Ma, ChemSusChem, 2013, 6, 411 CrossRef CAS PubMed.
- X. Zhang, X. Chen, S. Dong, Z. Liu, X. Zhou, J. Yao, S. Pang, H. Xu, Z. Zhang, L. Li and G. Cui, J. Mater. Chem. A, 2013, 1, 6067 Search PubMed.
- Q. W. Jiang, G. R. Li and X. P. Gao, Chem. Commun., 2009, 6720 RSC.
- G. Li, J. Song, G. Pan and X. Gao, Energy Environ. Sci., 2011, 4, 1680 CAS.
- G. H. Guai, M. Y. Leiw, C. M. Ng and C. M. Li, Adv. Energy Mater., 2012, 2, 334 CrossRef CAS PubMed.
- H. W. Zhou, Y. T. Shi, Q. S. Dong, Y. X. Wang, C. Zhu, L. Wang, N. Wang, Y. Wei, S. Y. Tao and T. L. Ma, J. Mater. Chem. A, 2014, 2, 4347 CAS.
- Y. Dou, G. Li and X. Gao, Phys. Chem. Chem. Phys., 2012, 14, 1339 RSC.
- S. H. Chang, M. D. Lu, Y. L. Tung and H. Y. Tuan, ACS Nano, 2013, 7, 9443 CrossRef CAS PubMed.
- C. W. Kung, H. W. Chen, C. Y. Lin, K. C. Huang, R. Vittal and K. C. Ho, ACS Nano, 2012, 6, 7016 CrossRef CAS PubMed.
- H. Sun, D. Qin, S. Huang, X. Guo, D. Li, Y. Luo and Q. Meng, Energy Environ. Sci., 2011, 4, 2630 CAS.
- J. H. Guo, Y. T. Shi, Y. T. Chu and T. L. Ma, Chem. Commun., 2013, 49, 10157 RSC.
- F. Gong, H. Wang, X. Xu, G. Zhou and Z. S. Wang, J. Am. Chem. Soc., 2012, 134, 10953 CrossRef CAS PubMed.
- F. Gong, X. Xu, Z. Q. Li, G. Zhou and Z. S. Wang, Chem. Commun., 2013, 49, 1437 RSC.
- J. H. Guo, Y. T. Shi, C. Zhu, L. Wang, N. Wang and T. L. Ma, J. Mater. Chem. A, 2013, 1, 11874 CAS.
- J. M. Yan, H. Z. Huang, J. Zhang, Z. J. Liu and Y. Yang, J. Power Sources, 2005, 146, 264 CrossRef CAS PubMed.
- R. Z. Yang, Z. X. Wang, J. Y. Liu and L. Q. Chen, Electrochem. Solid-State Lett., 2004, 7, A496 CrossRef CAS PubMed.
- M. Nath, A. Choudhury, A. Kundu and C. N. R. Rao, Adv. Mater., 2003, 15, 2098 CrossRef CAS PubMed.
- M. Nath and C. N. R. Rao, Angew. Chem., Int. Ed., 2002, 41, 3451 CrossRef CAS.
- K. B. Tang, Y. T. Qian, J. H. Zeng and X. G. Yang, Adv. Mater., 2003, 15, 448 CrossRef CAS PubMed.
- M. Bruchez, M. Moronne, P. Gin, S. Weiss and A. P. Alivisatos, Science, 1998, 281, 2013 CrossRef CAS.
- C. Ma, Y. Ding, D. Moore, X. Wang and Z. L. Wang, J. Am. Chem. Soc., 2004, 126, 708 CrossRef CAS PubMed.
- Y. Yang, X. J. Fan, G. Casillas, Z. W. Peng, G. D. Ruan, G. Wang, M. J. Yacaman and J. M. Tour, ACS Nano, 2014, 8, 3939 CrossRef CAS PubMed.
- J. X. Li, M. Z. Zou, L. Z. Chen, Z. G. Huang and L. H. Guan, J. Mater. Chem. A, 2014, 2, 10634 CAS.
- J. S. Lee, G. S. Park, S. T. Kim, M. L. Liu and J. Cho, Angew. Chem., 2013, 125, 1060 CrossRef PubMed.
- Z. H. Wen, S. Q. Ci, F. Zhang, X. L. Feng, S. M. Cui, S. Mao, S. L. Luo, Z. He and J. H. Chen, Adv. Mater., 2012, 24, 1399 CrossRef CAS PubMed.
- Y. C. Wang, D. Y. Wang, Y. T. Jiang, H. A. Chen, C. C. Chen, K. C. Ho, H. L. Chou and C. W. Chen, Angew. Chem., 2013, 125, 6826 CrossRef PubMed.
- Y. Hou, D. Wang, X. H. Yang, W. Q. Fang, B. Zhang, H. F. Wang, G. Z. Lu, P. Hu, H. J. Zhao and H. G. Yang, Nat. Commun., 2013, 4, 1583 CrossRef PubMed.
- L. Wang, Y. T. Shi, Y. X. Wang, H. Zhang, H. W. Zhou, Y. Wei, S. Y. Tao and T. L. Ma, Chem. Commun., 2014, 50, 1701 RSC.
- L. Wang, Y. T. Shi, H. Zhang, X. G. Bai, Y. X. Wang and T. L. Ma, J. Mater. Chem. A, 2014, 2, 15279 CAS.
- G. Kresse and J. Hafner, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 49, 14251 CrossRef CAS.
- G. Kresse and J. Furthmuller, Comput. Mater. Sci., 1996, 6, 15 CrossRef CAS.
- G. Kresse and J. Furthmuller, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169 CrossRef CAS.
- P. E. Blöchl, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 17953 CrossRef.
- X. Fang, T. Ma, M. Akiyama, G. Guan, S. Tsunematsu and E. Abe, Thin Solid Films, 2005, 472, 242 CrossRef CAS PubMed.
- M. X. Wu, X. Lin, T. H. Wang, J. S. Qiu and T. L. Ma, Energy Environ. Sci., 2011, 4, 2308 CAS.
- T. Harada, J. Phys. Soc. Jpn., 1998, 67, 1352 CrossRef CAS.
- A. Hauch and A. Georg, Electrochim. Acta, 2001, 46, 3457 CrossRef CAS.
- F. F. Volkenstein, The Electronic Theory of Catalysis on Semiconductors, Pergamon Press, Oxford, 1963 Search PubMed.
- B. X. Yuan, W. L. Luan and S. T. Tu, Dalton Trans., 2012, 772 RSC.
- W. X. Zhang, Z. H. Yang, J. H. Zhan, L. Yang, W. H. Yu, G. E. Zhou and Y. T. Qian, Mater. Lett., 2001, 47, 367 CrossRef CAS.
- P. Joshi, L. Zhang, Q. Chen, D. Galipeau, H. Fong and Q. Qiao, ACS Appl. Mater. Interfaces, 2010, 2, 3572 CAS.
- L. Yi, Y. Liu, N. Yang, Z. Tang, H. Zhao, G. Ma, Z. Sua and D. Wang, Energy Environ. Sci., 2013, 6, 835 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Theoretical lattice constants. XRD and SEM of FeTe2 with different reaction times; consecutive CVs of three iron chalcogenides CEs. See DOI: 10.1039/c5ra13147b |
| ‡ JiahaoGuo and Suxia Liang contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |