Removal of chlorpyrifos from waste water by wheat straw-derived biochar synthesized through oxygen-limited method†
Peifang Wang*,
Yayun Yin,
Yong Guo* and
Chao Wang
Key Laboratory of Integrated Regulation and Resource Development on Shallow Lakes, Ministry of Education, College of Environment, Hohai University, P.R. China. E-mail: pfwang2005@hhu.edu.cn; guoyong@hhu.edu.cn
First published on 11th August 2015
Abstract
Systematic studies have been performed for the first time to investigate the pyrolysis behavior of wheat straw and the adsorption mechanism of chlorpyrifos by wheat straw-derived biochar. FTIR and elemental analysis indicated that aromatic and hydrophobic substances are produced during the pyrolysis process of wheat straw. The BET results suggested that the pyrolysis temperature of wheat straw should be above 450 °C to acquire the biochar with a surface area above 60.0 m2 g−1. The adsorption experiments show that wheat straw-derived biochar at 750 °C (WS750) can effectively adsorb chlorpyrifos and the largest adsorption quantity is 16 mg g−1. The driving force for chlorpyrifos adsorption by WS750 is most likely attributed to the π⋯π stack between the aromatic ring of chlorpyrifos and these aromatic areas on WS750 surface. The adsorption behaviors follow the pseudo-second kinetic and Freundlich models. Recycle experiments show that the adsorption ability of WS750 can be recovered by washing with methanol. The present study shows that wheat straw-derived biochar can work as a highly effective and low-cost adsorbent to remove chlorpyrifos from waste water.
1. Introduction
China is a large agricultural country because it has nearly the 1/4 of the world population to support. For making sure the high yield of crops, such as wheat and rice, pesticides have been widely used to kill pests. As a broad spectrum organophosphate pesticide, the toxicity of chlorpyrifos is lower than that of other organophosphate pesticides, such as methamidophos and ammonium phosphate.1 Thus, chlorpyrifos has been used to replace these highly toxic organophosphate pesticides for killing agricultural pests. However, it has reported that chlorpyrifos can transfer into river from farmland, which is toxic to some species in water, e.g. frog and fish.1 Some methods have been developed to remove chlorpyrifos from water. Chishti et al. used microorganisms to degrade chlorpyrifos in water.2 Weston et al. suggested that enzymes could be applied to evaluate and reduce the toxicity of chlorpyrifos in water.3 TiO2 was used to photodegrade chlorpyrifos in water by Kanmoni et al.4 Adsorption is a commonly used method to remove these contaminants from water because of its simplicity and low cost.5 Presently, most of research has focused on the adsorption and desorption of chlorpyrifos in soil.6 Reports using adsorbents to remove chlorpyrifos from water are rare. Zhao et al.7 has found that the residual of drinking water treatment material can effectively adsorb chlorpyrifos in water with respect to paddy soil, which contains iron, aluminium hydroxide minerals and humic materials. In addition, the largest adsorption quantity is about 1.2 mg g−1.7 It is still necessary to develop low cost and high effective adsorbents for removing chlorpyrifos from waste water.As a large agricultural country, the wheat yield in China is very high,8 which leads to a large number wheat straw. The traditional way of treating wheat straw is to burn it in the field, which has resulted in serious air contamination.9 To reduce the environmental pollution and waste recycling, many methods have been developed to reuse wheat straw, in which using wheat straw to produce biochar is promising because biochar can be applied to soil remediation, carbon dioxide fixation and adsorbent due to its large surface area and high microporosity.10 Usually, wheat straw is converted to biochar in anoxic conditions under high temperatures.11 However, the cost is high because an inert gas, such as nitrogen, is needed to maintain anoxic conditions during the carbonization process. In addition, the requirement for gas tightness of a muffle furnace is also high. To lower the cost and simplify the carbonization procedure, the oxygen-limited method was developed to synthesize biochar, in which oxygen availability was restricted using a cover to close the feedback in a crucible.12 Sun et al. prepared biochar by an oxygen-limited method using aluminium foil to wrap the wheat straw during heating process.13 Using cover to close wheat straw in a crucible may be a better method to maintain oxygen-limited conditions than that using aluminium foil because the former is easier to use on a large-scale, can withstand higher temperatures and has better gas tightness. Furthermore, the surface area is a crucial parameter for evaluating the adsorption ability of biochar. Thus far, the relationship between the surface area and pyrolysis temperature of wheat straw-derived biochar has not been investigated.
Currently, studies on of wheat straw derived biochar have focused on using them to remediate the contaminated soil, such as to immobilize chlorobenzenes in soil,14 to replace peat in soilless substrates,15 and to adsorb cadmium cations in soil.16 No report using wheat straw-derived biochar to remove chlorpyrifos from water has been found.
Herein, detailed research has been for the first time performed to investigate the relationship between the surface area and pyrolysis temperature of wheat straw-derived biochar synthesized through an oxygen-limited method, in which the oxygen availability is restricted using a cover to close wheat straw in a crucible and the temperature was varied from 250 °C to 750 °C. The as-prepared biochar were then used to adsorb chlorpyrifos to evaluate their adsorption ability. The results show that wheat straw-derived biochar can effectively remove chlorpyrifos from water. This work is helpful for promoting the application of wheat straw-derived biochar to purify waste water.
2. Experimental section
2.1. Preparation of biochar
Wheat straw was first washed to remove mud and other impurities attached to its surface, followed by drying at 80 °C for overnight. The dried wheat straw was crushed into a powder through a disintegrator and then passed through a 20 mesh sieve. After that, the obtained wheat straw powder was placed into the full crucible and the volume of the crucible was 100 ml. Subsequently, the crucible was closed with a cover and heated in a furnace at 250 °C, 350 °C, 450 °C, 550 °C, 650 °C, and 750 °C for two hours each. The heating rate was 10 °C min−1. The acquired biochar samples were first washed with 1 mol L−1 HCl to remove the soluble minerals, which was followed by washing with deionized water to neutral state. These as-prepared samples were called WS250, WS350, WS450, WS550, WS650 and WS750, respectively.2.2. Characterization of samples
The thermal stability of wheat straw was characterized by thermogravimetric analysis (Netzsch STA 449 F1 Jupiter, Germany). The H/C ratio in WS750 was determined by elemental analysis on an elemental analyzer (Vario EL II, Elementar, Germany). The Fourier transform infrared spectra of WS250, WS350, WS450, WS550, WS650, and WS750 samples were acquired using Nexus 870 FT-IR instrument (USA). The surface areas of WS250, WS350, WS450, WS550, WS650 and WS750 samples were determined using a HD88, ASAP2020 micropore analyzer (USA). The morphology of WS750 was investigated using a JEM-2100 electron microscope (Japan). The zeta potentials of WS750 at different pH were performed using the Zetasizer Nano ZS (UK).The chlorpyrifos concentration was determined at a wavelength of 300 nm by High Performance Liquid Chromatography (HPLC, Waters e2696, USA) with a UV detector (Waters 2489) and a column (Bridge, 5 μm, 4.6 × 150 mm C18). The mobile phase used was the mixture of methanol and water (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 v
10 v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v), and the flow rate was 1 mL·min−1. The temperature of column was kept at 25 °C. The injected sample volume was 100 μL and the retention time was 3.9 min.
v), and the flow rate was 1 mL·min−1. The temperature of column was kept at 25 °C. The injected sample volume was 100 μL and the retention time was 3.9 min.
2.3. Adsorption experiments
A 2.5 mg biochar sample was weighed and placed into an EPA bottle and the bottle cap has Teflon gasket. The EPA bottle was purchased from Shanghai ANPEI Instrument Co. Ltd, China. Subsequently, 0.80 mg L−1 chlorpyrifos was placed into the EPA bottle, followed by rotation for 48 hours with at rotation rate of 70 rpm at room temperature under dark conditions. Three parallel experiments were performed for each biochar sample. After the adsorption experiments were finished, the supernatants in these EPA bottles were taken to determine the adsorption effect with HPLC method.
3. Results and discussion
A detailed analysis was first performed to investigate the surface areas of these as-prepared biochar samples. According to Table 1, the pore volumes and pore diameters of WS250 and WS350 are not detected by the micropore analyzer, suggesting that pores had not formed in these two samples. Accordingly, the surface areas of WS250 and WS350 are only 0.114 m2 g−1 and 0.432 m2 g−1, respectively. The pore volumes and pore diameters of WS450, WS550, WS650 and WS750 increased with the increasing pyrolysis temperature, and the surface area increased from 63.5 m2 g−1 of WS450 to 467 m2 g−1 of WS750. One obvious increase in surface area can be found according to Table 1: it was between 350 °C and 450 °C, in which the surface area increased from 0.432 m2 g−1 to 63.5 m2 g−1. This suggests that a pyrolysis temperature of at least 450 °C is needed to use wheat straw to produce biochar with an oxygen-limited method because a significant surface area can just be acquired when pyrolysis temperature is above 450 °C. As far as we know, this is first clear description of the relationship between the surface area and the pyrolysis temperature of wheat straw-derived biochar, which is helpful for researchers to choose an appropriate pyrolysis temperature to produce wheat straw-derived biochar with a large surface area.| Biochar | T (°C) | BET (m2 g−1) | Pore volume (cm3 g−1) | Pore diameter (nm) | Ash (%) | Yield (%) |
|---|---|---|---|---|---|---|
| WS250 | 250 | 0.114 | — | — | 1.9 | 67.4 |
| WS350 | 350 | 0.432 | — | — | 5.7 | 36.9 |
| WS450 | 450 | 63.5 | 0.01 | 1.83 | 8.5 | 29.9 |
| WS550 | 550 | 303 | 0.16 | 2.13 | 9.7 | 26.1 |
| WS650 | 650 | 336 | 0.18 | 2.14 | 11 | 24.9 |
| WS750 | 750 | 467 | 0.26 | 2.19 | 12 | 21.5 |
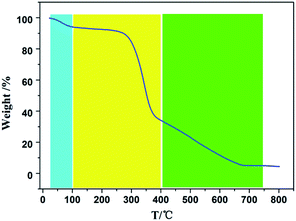
For a deep understanding of the relationship between surface area and pyrolysis temperature of wheat straw-derived biochar, thermogravimetric analysis (TGA) was performed to investigate the pyrolysis behavior of wheat straw. It was reported that the components of wheat straw are mainly lignin, cellulose and hemicellulose,17 in which cellulose and hemicellulose decompose from 350 °C to 400 °C, while lignin will decompose at temperatures higher than 400 °C.17,18 According to Fig. 1, three weight loss intervals were found from the TGA curve of wheat straw. The first one ranged from 25 °C to 100 °C, which is from the loss of the adsorbed water in wheat straw. The second one starts from 100 °C to 400 °C, being due to the thermal decomposition of cellulose and hemicellulose.17,18 The third one ranged from 400 °C to 700 °C, being attributed to the thermal decomposition of lignin.17,18 By comparing Table 1 and Fig. 1, one can deduce that the thermal decomposition of cellulose and hemicellulose will result in the formation of pore, and is most likely responsible for the abrupt increase in surface areas from WS350 to WS450. Furthermore, the surface areas from WS450 to WS750 continued to increase with increasing pyrolysis temperature, which may be from the contribution of the pyrolysis of lignin at temperatures higher than 450 °C. So, the TGA result of wheat straw accounts well for the observed surface area results of wheat straw-derived biochar samples.
FTIR analysis was applied further to investigate the pyrolysis process of wheat straw (Fig. S1†). The broad peak around 3412 cm−1 and the peak at 2920 cm−1 are from the vibrations of O–H and aliphatic C–H2 groups, respectively, while the peak at 1573 cm−1 is assigned to the vibration of the aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond.19 According to Fig. S1,† the peaks of 3412 cm−1 and 2920 cm−1 gradually disappear, while the peak of 1573 cm−1 gradually appears. This suggests that the pyrolysis of wheat straw with increasing pyrolysis temperature will result in the formation of the aromatic and hydrophobic substances. In addition, the peak around 1100 cm−1 in the FTIR curve of WS750 is from the contribution of C–O and C–O–C groups,19 implying that there are still some oxygen-containing functional groups on the surface of the WS750 sample.
C bond.19 According to Fig. S1,† the peaks of 3412 cm−1 and 2920 cm−1 gradually disappear, while the peak of 1573 cm−1 gradually appears. This suggests that the pyrolysis of wheat straw with increasing pyrolysis temperature will result in the formation of the aromatic and hydrophobic substances. In addition, the peak around 1100 cm−1 in the FTIR curve of WS750 is from the contribution of C–O and C–O–C groups,19 implying that there are still some oxygen-containing functional groups on the surface of the WS750 sample.
Chlorpyrifos is a broad spectrum organophosphate pesticide, which is toxic to some species in water, e.g. frog and fish.1 According to our knowledge, wheat straw-derived biochar has not been used to adsorb chlorpyrifos from waste water. Herein, we first investigated the solubility of chlorpyrifos in water because it does not dissolve easy in water. According to Fig. S2,† chlorpyrifos can dissolve well in water when the concentration is smaller than 1.2 mg L−1. So, the concentrations of chlorpyrifos in this work were all smaller than 1.2 mg L−1 to avoid the possible self-aggregation of chlorpyrifos. Then, the adsorption ability of WS250–WS750 samples for chlorpyrifos was compare. Based on Fig. 2, WS750 has the highest adsorption ability for chlorpyrifos in all samples (about 12 mg g−1) because the aromatic and hydrophobic degree of WS750 is the highest among all samples and chlorpyrifos has an aromatic ring as well. Therefore, WS750 is chosen as a model compound of wheat straw-derived biochar and further studies have been performed to investigate the adsorption mechanism of chlorpyrifos by WS750.
 | ||
| Fig. 2 Comparison of chlorpyrifos adsorption by WS250–WS750 samples. The concentration of chlorpyrifos is 0.80 mg L−1. | ||
Further characterizations of WS750 were first carried out. According to the TEM image (Fig. S3†), the structure of WS750 is loose and there are many pores on its surface, which is consistent with the surface area result of WS750 (Table 1). The H/C ratio is usually used to characterize the aromatization degree of a biochar sample. For example, H/C ratios between 0.13 and 0.37 suggest that the biochar samples have a highly aromatic structure.19 The H/C ratio of WS750 is 0.25, supporting that the aromatization degree of WS750 is high, which is line with the FTIR analysis result of WS750. The inorganic constituents in WS750 were also analysed by EDS. Based on Fig. S4,† the contents of O (55%, weight percentage) and Si (34%) were significant higher than the remaining elements, such as Na (0.08%), Al (0.38%), K (1.1%), Ca (3.6%), S (1.4%) and P (1.1%), suggesting that the inorganic constituent in WS750 is mainly SiO2.
The adsorption kinetics of chlorpyrifos by WS750 has been done by detecting the concentration of chlorpyrifos taken at 0.5, 1, 2, 4, 6, 8, 10, 12, 24, 36, 48, 60, and 72 hours, respectively. From Fig. 3, the adsorption of chlorpyrifos by WS750 includes two adsorption periods: fast adsorption and slow adsorption. The fast adsorption period is from 0 hour to 12 hours, and nearly 70% chlorpyrifos is adsorbed in this period. Slow adsorption period ranges from the 12 hours to 48 hours and the rest 30% chlorpyrifos is adsorbed. After 48 hours, the adsorption equilibrium arrives. Pseudo-first-order and pseudo-second-order models have been used to analyse the kinetic adsorption of chlorpyrifos by WS750 (Fig. S5 and Table S1†).20 The experimental adsorption quantity of chlorpyrifos is 12 mg g−1, while the adsorption quantity of chlorpyrifos from the pseudo-first-order model and pseudo-second-order model are 11.080 ± 0.560 mg g−1 and 12.195 ± 0.593 mg g−1, respectively. Furthermore, the R2 from pseudo-second-order model is 0.991, while the R2 from pseudo-first-order model is 0.841. All of this suggests that the adsorption of chlorpyrifos by WS750 follows the pseudo-second-order kinetics. This implies that the adsorption sites on WS750 surface are not homogeneous, which is consistent with the FTIR and TEM results. According to the FTIR and BET results (Fig. S1† and Table 1), WS750 has an aromatic surface and cavity with a diameter larger than 2.19 nm. Fast adsorption is most likely from the adsorption of chlorpyrifos on the aromatic areas of the WS750 surface and the mouth of the cavity in WS750. Slow adsorption is possible from the transfer of chlorpyrifos from the mouth to the inside of the cavity.
 | ||
| Fig. 3 The chlorpyrifos adsorption by WS750 at 0.5, 1, 2, 4, 6, 8, 10, 12, 24, 36, 48, 60 and 72 hours. The concentration of chlorpyrifos is 0.80 mg L−1. | ||
The adsorption isotherm of chlorpyrifos by WS750 has also been investigated. According to Fig. 4, the adsorption quantity of chlorpyrifos by WS750 increases with increasing concentration of chlorpyrifos, and the largest adsorption quantity was around 16 mg g−1. The Freundlich method is usually used to describe the adsorption behavior of biochar.21 From Fig. S6 and Table S2,† the correlation coefficient R2 is 0.968, implying that Freundlich method can describe well the adsorption behavior of chlorpyrifos by WS750. The K and 1/n values from Freundlich fitting results are 30.265 ± 4.852 and 0.413 ± 0.0187, respectively, implying that WS750 has strong affinity for chlorpyrifos. There is an aromatic ring in chlorpyrifos, and some areas on WS750 surface are aromatic. Therefore, the affinity between chlorpyrifos and WS750 possibly from the π⋯π interaction between the aromatic ring of chlorpyrifos and these aromatic areas on WS750 surface.22–25
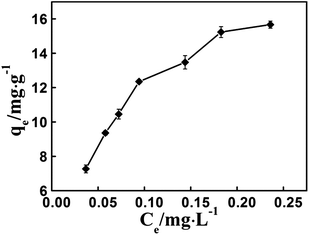 | ||
| Fig. 4 The adsorption isotherm of chlorpyrifos by WS750. The concentration of chlorpyrifos ranges from 0.40 mg L−1 to 1.2 mg L−1. | ||
There is an inorganic component in WS750 and EDS confirmed that the inorganic component is mainly SiO2. An adsorption experiment was performed to clarify the role of the inorganic component in the adsorption of chlorpyrifos by WS750. The acquired result shows that the inorganic component in WS750 does not adsorb chlorpyrifos.
CaCl2 has been added in the diluted chlorpyrifos solution to maintain a constant ionic strength.7 Series adsorption experiments were done to investigate the influence of CaCl2 concentration on the adsorption of chlorpyrifos by WS750. According to Fig. 5, the adsorption quantity of chlorpyrifos by WS750 decreases with increasing CaCl2 concentration. This may be because these increased ions (Ca2+ and Cl−) can occupy the adsorption sites on the surface of WS750 through an ion⋯π interaction,7,24,25 which leads to a decrease in chlorpyrifos adsorption.
 | ||
| Fig. 5 The influence of CaCl2 concentration on the adsorption of chlorpyrifos by WS750. The concentration of chlorpyrifos is 0.80 mg L−1. | ||
A detailed adsorption experiments have also been performed to investigate the influence of pH on the adsorption of chlorpyrifos by WS750. According to Fig. 6, the adsorption quantity decreases with the increase of pH. For a better understanding of the experimental observation, the charge in surface of WS750 at different pH was also investigated. The surface charge of WS750 at basic situations was not investigated because chlorpyrifos will hydrolyse under basic conditions. Based on Fig. 7, the surface charges in WS750 change from positive to negative with increasing pH from 1.27 to 5.18. The zero point charge of the WS750 surface was around pH3.30. The change in charge on the WS750 surface at different pH is most likely due to the protonation/deprotonation of oxygen-containing functional groups on the WS750 surface. From Fig. S1,† the peak around 1100 cm−1 supports the existence of C–O and C–O–C groups.19 In most cases, the cation is most likely to interact with aromatic ring than anion.26,27 So, it is easy to explain why WS750 with a positive surface has a stronger adsorption for chlorpyrifos than that with negative surface. In addition, there are O atoms in chlorpyrifos. This means that a possible hydrogen bonding interaction between the proton on WS750 surface and O atoms in chlorpyrifos may also exist,23 which will further increase the adsorption of chlorpyrifos by WS750 under low pH conditions.
 | ||
| Fig. 6 The influence of pH on the adsorption of chlorpyrifos by WS750. The concentration of chlorpyrifos is 0.80 mg L−1. | ||
A possible adsorption mechanism was proposed to explain the adsorption behavior of chlorpyrifos by WS750. FTIR, elemental analysis and BET results show that some areas on WS750 surface are aromatic and hydrophobic, while the diameter of cavity in the inside of WS750 is about 2.19 nm. There is an aromatic ring in chlorpyrifos and its size is less than 2.19 nm. Based on the fitting results from Freundlich and pseudo-second-order models, WS750 has strong attraction for chlorpyrifos and the adsorption behavior include two periods: fast adsorption and slow adsorption. From Scheme 1, the π⋯π interaction between the aromatic ring of chlorpyrifos and the aromatic areas on WS750 surface may be responsible for the effective adsorption of chlorpyrifos by WS750.22–25 Fast adsorption is most likely from the adsorption of chlorpyrifos on the aromatic areas on the WS750 surface and the mouth of the cavity in WS750, while slow adsorption is possible from the transfer of chlorpyrifos from the mouth to inside of the cavity.
 | ||
| Scheme 1 The possible adsorption mechanism of chlorpyrifos by wheat straw-derived biochar synthesized using an oxygen-limited method. | ||
The recycle adsorption experiments of WS750 have also been performed to explore the possibility of using WS750 as an adsorbent to clean waste water in real situations. The adsorption ability of WS750 was recovered by washing with methanol. According to Fig. S7,† the adsorption quantity of WS750 decreased to 7.5 mg g−1 in the second time from the 12 mg g−1 in the first time, suggesting that washing can just recover 63% of the adsorption ability of WS750. Interestingly, the adsorption ability of WS750 in the third time was similar to that in the second time. According to Fig. 3 and Scheme 1, 70% chlorpyrifos is adsorbed by WS750 in the fast adsorption period. Therefore, the chlorpyrifos adsorbed on WS750 surface can be washed easily by methanol, but the disadsorption of chlorpyrifos in the cavity is not easy. However, the adsorption quantity (7.5 mg g−1) is still higher than the reported one (1.2 mg g−1),7 and the recovery method is very simple. So, it is feasible to use WS750 as an adsorbent for purifying waste water.
4. Conclusions
In summary, systematic studies have been performed to investigate the pyrolysis behavior of wheat straw and the adsorption mechanism of chlorpyrifos by wheat straw-derived biochar. The BET results suggest that the pyrolysis temperature of using wheat straw to produce biochar should be above 450 °C. FTIR and elemental analysis results support that the pyrolysis of wheat straw will lead to the appearance of aromatic and hydrophobic substances. The adsorption experiments show that WS750 can effectively adsorb chlorpyrifos and the largest adsorption quantity is 16 mg g−1. The driving force for chlorpyrifos adsorption by WS750 is mainly from the π⋯π interaction between the aromatic ring of chlorpyrifos and the aromatic areas on the surface of WS750. Recycle experiments show that the adsorption ability of WS750 can be recovered by washing with methanol. The present study will be helpful for promoting the application of wheat straw-derived biochar for the purification of waste water.Acknowledgements
Financial support was provided by the National Science Funds for Creative Research Groups of China (No. 51421006), Program for Changjiang Scholars and Innovative Research Team in University (No. IRT13061), National Science Fund for Distinguished Young Scholars (No. 51225901), the Fundamental Research Funds for the Central Universities (2014B18614), Natural Science Foundation of Jiangsu Province (SBK2015021389), A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.Notes and references
- D. L. Eaton, R. B. Daroff, H. Autrup, J. Bridges, P. Buffler, L. G. Costa, J. Coyle, G. McKhann, W. C. Mobley, L. Nadel, D. Neubert, R. Schulte-Hermann and P. S. Spencer, Crit. Rev. Toxicol., 2008, 38, 1 CrossRef CAS PubMed.
- Z. Chishti, S. Hussain, K. R. Arshad, A. Khalid and M. Arshad, J. Environ. Manage., 2013, 114, 372 CrossRef CAS PubMed.
- D. P. Weston and C. J. Jackson, Environ. Sci. Technol., 2009, 43, 5514 CrossRef CAS.
- V. G. G. Kanmoni, S. Daniel and G. A. G. Raj, React. Kinet., Mech. Catal., 2012, 106, 325 CrossRef CAS.
- J. Zolgharnein, A. Shahmoradi and J. Ghasemi, Clean: Soil, Air, Water, 2011, 39, 1105 CrossRef CAS PubMed.
- S. Y. Gebremariam, M. W. Beutel, D. R. Yonge, M. Flury and J. B. Harsh, Rev. Environ. Contam. Toxicol., 2012, 215, 123 Search PubMed.
- Y. Y. Zhao, C. H. Wang, L. A. Wendling and Y. S. Pei, J. Agric. Food Chem., 2013, 61, 7446 CrossRef CAS PubMed.
- D. R. Wu, Q. Yu, C. H. Lu and H. Hengsdijk, Eur. J. Agron., 2006, 24, 226 CrossRef.
- X. H. Li, S. X. Wang, L. Duan, J. M. Hao, C. Li, Y. S. Chen and L. Yang, Environ. Sci. Technol., 2007, 41, 6052 CrossRef CAS.
- J. J. Manyaà, Environ. Sci. Technol., 2012, 46, 7939 CrossRef PubMed.
- Y. Song, F. Wang, Y. R. Bian, F. O. Kengara, M. Y Jia, Z. B. Xie and X. Jiang, J. Hazard. Mater., 2012, 391, 217 Search PubMed.
- B. L. Chen, D. Zhou and L. Zhu, Environ. Sci. Technol., 2008, 42, 5137 CrossRef CAS.
- J. N. Sun, B. C. Wang, G. Xu and H. G. Shao, Ecol. Eng., 2014, 62, 43 CrossRef PubMed.
- Y. Song, F. Wang, F. O. Kengara, X. L. Yang, C. G. Gu and X. Jiang, J. Agric. Food Chem., 2013, 61, 4210 CrossRef CAS PubMed.
- S. F. Vaughn, J. A. Kenar, A. R. Thompson and S. C. Petersonb, Ind. Crops Prod., 2013, 51, 437 CrossRef CAS PubMed.
- D. Y. Xu, Y. Zhao, K. Sun, B. Gao, Z. Y. Wang, J. Jin, Z. Y. Zhang, S. F. Wang, Y. Yan, X. T. Liu and F. C. Wu, Chemosphere, 2014, 111, 320 CrossRef CAS PubMed.
- M. I. Jahirul, M. G. Rasul, A. A. Chowdhury and N. Ashwath, Energies, 2012, 5, 4952 CrossRef CAS PubMed.
- H. P. Yang, R. Yan, H. P. Chen, D. H. Lee and C. G. Zheng, Fuel, 2007, 86, 1781 CrossRef CAS PubMed.
- Q. L. Fang, B. L. Chen, Y. J. Lin and Y. T. Guan, Environ. Sci. Technol., 2014, 48, 279 CrossRef CAS PubMed.
- F. Zhang, X. Wang, D. X. Yin, B. Peng, C. Y. Tan, Y. G. Liu, X. F. Tan and S. X. Wu, J. Environ. Manage., 2015, 153, 68 CrossRef CAS PubMed.
- L. P. Lou, B. B. Wu, L. Wang, L. Luo, X. H. Xu, J. A. Hou, B. Xun, B. L. Hu and Y. X. Chen, Bioresour. Technol., 2011, 102, 4036 CrossRef CAS PubMed.
- C. X. Ren, L. X. Cai, C. Chen, B. Tan, Y. J. Zhang and J. Zhang, J. Mater. Chem. A, 2014, 2, 9015 CAS.
- M. À. Olivella, C. Bazzicalupi, A. Bianchi, N. Fiol and I. Villaescusa, Chemosphere, 2015, 119, 863 CrossRef CAS PubMed.
- X. X. Chen and B. L. Chen, Environ. Sci. Technol., 2015, 49, 6181 CrossRef CAS PubMed.
- M. M. Watt, M. S. Collins and D. W. Johnson, Acc. Chem. Res., 2013, 46, 955 CrossRef CAS PubMed.
- B. Sharma, H. K. Srivastava, G. Gayatri and G. N. Sastry, J. Comput. Chem., 2015, 36, 529 CrossRef CAS PubMed.
- H. R. Masoodi, S. Bagheri, M. Mohammadi, M. Zakarianezhad and B. Makiabadi, Chem. Phys. Lett., 2013, 588, 31 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Regents, the FTIR, TEM, EDS and fitting results are provided. See DOI: 10.1039/c5ra10487d |
| This journal is © The Royal Society of Chemistry 2015 |