Modified Kagan's amide: synthesis and application as a chiral solvating agent for hydrogen-bonding based chiral discrimination in NMR†
Nilesh
Jain
,
Ravi B.
Patel
and
Ashutosh V.
Bedekar
*
Department of Chemistry, Faculty of Science, M. S. University of Baroda, Vadodara 390 002, India. E-mail: avbedekar@yahoo.co.in; Tel: +91 265 2795552
First published on 15th May 2015
Abstract
A modified Kagan's amide, N-((S)-1-(3,5-bis(trifluoromethyl)phenyl)ethyl)-3,5-dinitrobenzamide [(S)-2] has been designed, synthesized and screened as a Chiral Solvating Agent (CSA) for discrimination of optically active substrates. The proposed mode of action for recognition of chiral isomers of analytes by CSA is based on hydrogen-bonding. The 1H NMR signals of the two isomers of chiral amides, sulfoxides, α-substituted acids, α-hydroxy ketone, epoxy ketone and N-protected amino acids with (S)-2 were well resolved for practical applications of determination of optical purity. The CSA was also screened for detection of separation of signals in 19F NMR.
Introduction
The advent of the chemistry of chiral molecules in recent years has been triggered by discoveries of their special properties in many fields. The specific properties of chiral molecules are often linked with a particular optical isomer and drastically differ with its purity. In some cases different chiral isomers have been found to posses completely divergent properties. Hence, establishing the enantiomeric purity and absolute configuration of chiral compounds is a critical factor in the study and applications of optically active molecules. The optical character of the chemical sample can be analyzed by different techniques ranging from chromatography,1 mass spectrometry,2 IR, UV and fluorescence spectroscopy,3 circular dichroism and electrophoresis4 etc. These techniques need a certain type of structural requirement or depend on the presence of a certain functional group in the analyte or need different accessories on the analytical machine, such as special chiral columns for the chromatographic techniques. Recently some biochemical methods are also developed to determine the enantiomeric excess of the chiral products.5The technique of Nuclear Magnetic Resonance (NMR) spectroscopy, with its highly advance and sensitive mode of analysis, also offers an alternative methods of quick and accurate determination of optical purity of chiral molecules.6 However, the standard NMR analysis of chiral molecules in achiral environment cannot differentiate the signals of the two enantiomers. Few isolated attempts to use chiral solvents in NMR analysis have met with limited success.7 For the detectable NMR discrimination the enantiomers need to be converted to diastereomers, either permanently by covalent bond formation or temporarily by non-covalent, supramolecular interactions. One such classical technique involves in situ preparation of diastereomeric lanthanide chelate complexes.8 This can also be done by the use of chiral derivatizing agents (CDA)9 for formation of diastereomeric compounds, either prior to the analysis or for the in situ in NMR tube analysis.10 Similarly the diastereomer formation for NMR analysis may also be done by in situ complex formation with chiral solvating agent (CSA) during the measurement of the spectra.11 Although examination of 1H NMR is more widely investigated, the researchers have also focused on targeting the analysis of other NMR active nuclei to determine optical purity using this protocol.12–16 These techniques involves non-covalent interactions between the test sample and the chirally pure molecule of CSA. These are primarily hydrogen-bonding, π–π stacking, CH–π interactions, van der Waals interactions etc. The efficiency of a given CSA to recognize two isomers of chiral analyte depends on combination of these forces and hence they are often selective and case sensitive in their action. The specific nature of the action of each CSA for different substrates necessitates the need to have a library to scan for new analytes for the NMR analysis.
Chiral amide of 3,5-dinitro benzoic acid 1a reported by Kagan is one of the oldest and well studied CSA for efficiently discriminating several types of molecules by NMR analysis.17 This is a simple molecule with basic functional units present to enable hydrogen-bonding and π–π interactions with substrates for tight complex formations in NMR conditions. These type of compounds were first developed by Pirkle for the Whelk-O type materials for separation of chiral compounds on HPLC columns.18 Over the years few more derivatives of Kagan's amide have been explored with good success in molecular recognition of chiral compounds (Chart 1).19 The range of chiral analytes successfully screened with these CSAs include amides,19c sulfoxides,17b,17d multifunctional tert-alcohols,19d and phosphine oxides.17c
Generally accepted mode of interaction between the two molecules involve hydrogen-bonding between the CSA and the analyte, N–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) X (X = C or S of analyte), and the π–π interaction between the aromatic units.19d It is also proposed that the analyte fits between the cleft type conformation created by perpendicularly arranged dinitro benzoyl and the naphthalene rings in case of 1b,19c similar type of explanations are offered for the other derivatives 1c and 1d.20 The proposed interactions were also corroborated by up-field and down-field shifts of appropriate examples in the 1H NMR experiments.
X (X = C or S of analyte), and the π–π interaction between the aromatic units.19d It is also proposed that the analyte fits between the cleft type conformation created by perpendicularly arranged dinitro benzoyl and the naphthalene rings in case of 1b,19c similar type of explanations are offered for the other derivatives 1c and 1d.20 The proposed interactions were also corroborated by up-field and down-field shifts of appropriate examples in the 1H NMR experiments.
Result and discussion
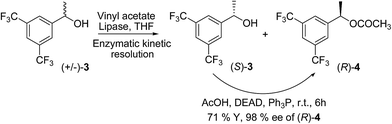
Based on these proposed models (A in Fig. 1) we suggest to introduce a modification in Kagan's amide where the chiral amine portion will possess an aromatic ring with two strongly electron withdrawing groups. With this modification the hydrogen attached to the nitrogen of the amide group of CSA will be more electron deficient due to the inductive effect and may form stronger hydrogen bonding with the carbonyl of the test sample (B in Fig. 1). We propose to introduce two trifluoromethyl groups at the meta positions of aromatic ring at the amine portion of the amide unit keeping the chiral centre unchanged. Our proposed model compound 2 has been known in the literature18c and has been employed as a support in chiral phase HPLC analysis. We present the synthesis and evaluation of 2 as CSA for determining chiral purity of suitable range of substrates by 1H NMR analysis.The synthesis of chiral 2 was based on condensation of the optically pure 1-(3,5-bis(trifluoromethyl)phenyl)ethanamine with 3,5-dinitrobenzoyl chloride under suitable conditions. Separation of isomers of optically pure alcohols using bio-catalytic selective methods is a well established protocol. Several studies have been conducted for elegant separation of alcohols based on kinetic resolution methods, where one of the enantiomers undergoes esterification while the other remains unaffected.21 As a part of our ongoing project we have separated isomers of chiral roof shape alcohols22 using enzyme mediated resolution process. Some of these alcohols have been converted to roof shape chiral amines and screened as CSAs for determination of optical purity of α-substituted acids.22b In the present synthesis we have accessed optically pure 1-(3,5-bis(trifluoromethyl)phenyl)ethanol 3 from its racemic sample via enzyme mediated transesterification process and the alcohol was converted to its amine by usual steps. The enzyme mediated process to resolve (±)-3 was conducted with vinyl acetate as the acyl source in presence of immobilized lipase (Scheme 1). The optically pure 3, which is an intermediate of aprepitant, a potent and orally active antagonist of human neurokinin receptor, has also been accessed by similar process.23 The improved and standardized parameters of the enantiomer separation study have been summarized in Table 1.
| No. | Conditions | % ee of 4b (% yield) | % ee of 3c (% yield) | C | E | ||
|---|---|---|---|---|---|---|---|
| Acyl donor | Solvent | Time (h) | |||||
| a All the reactions were run at r.t. b Determined by HPLC by converting to alcohol 3. c Determined by HPLC. d Trace reaction, not isolated. e No reaction. IPA = iso-propenyl acetate, VA = vinyl acetate, EA = ethyl acetate, BA = butyl acetate. All experiments were run with Novozyme-435. | |||||||
| 1 | IPA | THF | 72 | >99 (38) | 86 (37) | 46 | >200 |
| 2 | VA | THF | 72 | >99 (40) | 89 (45) | 47 | >200 |
| 3 | EA | THF | 72 | —d | — | — | — |
| 4 | — | EA | 72 | 94 (16) | 32 (75) | 25 | 44 |
| 5 | BA | THF | 72 | —e | — | — | — |
| 6 | IPA | THF | 84 | >99 (40) | 92 (35) | 48 | >200 |
| 7 | VA | THF | 84 | >99 (43) | >99 (41) | 50 | >200 |
However, the kinetic resolution of chiral alcohols cannot furnish either compound in more than 50% chemical yield in optically pure form. To overcome this difficulty a strategy of combination of enzymatic resolution and Mitsunobu reaction24 has been developed by few groups.25 The Mitsunobu reaction involves direct substitution of alcohols with new nucleophiles in presence of triphenyl phosphine and diethyl azodicarboxylate (DEAD) with complete inversion of configuration. In the above reaction (Scheme 1) the “R” isomer of the alcohol 3 was selectively converted to its acetate (R)-4 with excellent selectivity and moderate yield. The unaffected alcohol (S)-3 was also left in high optical purity. In the present effort the same reaction was conducted in presence of acetic acid under Mitsunobu conditions, where the acetate ion acts as new nucleophile and the unreacted alcohol (S)-3 was converted selectively to (R)-4 with complete inversion in overall good chemical yield (Scheme 2).
Thus a practical process was developed to access optically pure (R)-4, which was converted to chiral alcohol (R)-3 under the acidic hydrolysis conditions (Scheme 3).
Optically pure alcohol (R)-3 was then converted to corresponding amine (S)-6 (Scheme 4). This operation involved inversion of configuration by its reaction with phthalimide, Ph3P and DEAD in dry tetrahydrofuran, furnishing the corresponding protected amine (S)-5, which was subsequently deprotected with hydrazine.26 The amine (S)-6 (ref. 27) was not isolated but converted directly to the desired amide (S)-2 by treatment with 3,5-dinitrobenzoyl chloride. Similarly (S)-6 was also converted to (S)-7 by treatment with benzoyl chloride.
The single crystal analysis of 1b indicated a cleft-like arrangement caused by having the 3,5-dinitrobenzoyl group placed orthogonal to the naphthalene plane.19c The selective recognition of the two isomers of chiral amide analyte was attributed to this arrangement and was proposed to be responsible for the supramolecular interactions. We could also grow a single crystal of (S)-2 and its X-ray diffraction analysis was performed (Fig. 2).28 We observed that the angle between the planes passing through the two aromatic rings bisecting each other at an angle of 119.6° making the arrangement of the aromatic rings quite open compared to 1b (where the same angle was noted to be 99.9°) The intramolecular hydrogen bonding N–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C was detected with the bond distance of 2.104 Å.
C was detected with the bond distance of 2.104 Å.
Having prepared two derivatives of modified CSAs, (S)-2 and (S)-7, we next screened them to test their ability to discriminate the signals of chiral amide analytes. We made three types of the chiral amides Ph*CH(CH3)NHCOR (8a–8m) in the unequal ratio of its enantiomers and tested them with CSAs (Table 2). The first set of amides was made with aromatic acid moieties being phenyl, naphthyl and anthryl (8a–8c). The second set where the phenyl was substituted with electron donating or withdrawing groups (8d–8i). The third set of amides was made where the alkyl acids were condensed with α-methyl benzyl amine (8j–8m). The recognition study was conducted in CDCl3 (400 MHz; 10 mM concentration; ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) targeting two protons CαH of Ph*CH(CH3)NHCOR and methyl protons attached to the chiral center of Ph*CH(CH3)NHCOR. As can be seen there is not much difference in case of benzene and naphthalene derivatives (8a and 8b) while the latter signals were much resolved in case of anthracene derivative (8c). When the aromatic ring is attached with electron withdrawing group the degree of induced chemical shift (Δδ) and nonequivalences (ΔΔδ) for both sets of protons was observed to be marginally on the lower side (8d, 8f and 8p) while with electron donating group attached showed considerably enhance shifts in both the parameters (8g and 8i). In case of alkyl derivatives a pattern was observed where both the parameters were seen to increase with increase in the size of R group (8j and 8m). Higher values were seen in case of pivaloyl (8l) and adamantly (8m–8o) supporting this trend (Table 3). The adamantly amide prepared from naphthyl ethyl amide (8n) showed remarkable separation of signals with high nonequivalences of 0.502. The pattern observed for the second set of amide analytes was consistent with the proposed hydrogen bonding model (Fig. 1), where the electron donating groups on the amide (ArCO) would make the oxygen of carbonyl more electron rich and hence should favor the interaction by becoming better hydrogen bond acceptor. The opposite phenomena may account for the lower values in case of electron withdrawing substituents.
1) targeting two protons CαH of Ph*CH(CH3)NHCOR and methyl protons attached to the chiral center of Ph*CH(CH3)NHCOR. As can be seen there is not much difference in case of benzene and naphthalene derivatives (8a and 8b) while the latter signals were much resolved in case of anthracene derivative (8c). When the aromatic ring is attached with electron withdrawing group the degree of induced chemical shift (Δδ) and nonequivalences (ΔΔδ) for both sets of protons was observed to be marginally on the lower side (8d, 8f and 8p) while with electron donating group attached showed considerably enhance shifts in both the parameters (8g and 8i). In case of alkyl derivatives a pattern was observed where both the parameters were seen to increase with increase in the size of R group (8j and 8m). Higher values were seen in case of pivaloyl (8l) and adamantly (8m–8o) supporting this trend (Table 3). The adamantly amide prepared from naphthyl ethyl amide (8n) showed remarkable separation of signals with high nonequivalences of 0.502. The pattern observed for the second set of amide analytes was consistent with the proposed hydrogen bonding model (Fig. 1), where the electron donating groups on the amide (ArCO) would make the oxygen of carbonyl more electron rich and hence should favor the interaction by becoming better hydrogen bond acceptor. The opposite phenomena may account for the lower values in case of electron withdrawing substituents.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1]d
1]d
| No. | Amide 8 | ArCHMeNHCOR | ArCHMeNHCOR | ||
|---|---|---|---|---|---|
| Δδ | ΔΔδ | Δδ | ΔΔδ | ||
a Not resolved.
b With (S)-7.
c Complex pattern.
d All NMR recorded at 400 MHz in CDCl3 at 10 mM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1).
|
|||||
| 1 |

|
−0.086 | 0.060 | −0.018 | 0.025 |
| 2 |
|
−0.084 | 0.063 | −0.018 | 0.022 |
| 3 |
|
—a | —a | −0.049 | 0.045 |
| 4 |

|
−0.077 | 0.043 | −0.025 | 0.015 |
| 5 |

|
−0.072 | 0.044 | −0.012 | 0.017 |
| 6 |

|
−0.046 | —a | −0.014 | 0.018 |
| 7 |

|
−0.090 | 0.059 | −0.021 | 0.024 |
| 8 |

|
−0.091 | 0.059 | −0.019 | 0.025 |
| 9 |

|
−0.067 | 0.076 | −0.016 | 0.030 |
| 10 |

|
−0.110 (−0.004)b | 0.081 (—a,b) | −0.028 (−0.004)b | 0.034 (—a,b) |
| 11 |

|
−0.154 | 0.108 | −0.046 | 0.047 |
| 12 |

|
−0.158 (−0.007)b | 0.156 (—a,b) | −0.047 (−0.004b) | 0.057 (—a,b) |
| 13 |

|
−0.174 | 0.163 | −0.018 | 0.059 |
| 14 |

|
−0.39 | 0.502 | −0.212 | 0.241 |
| 15 |

|
−0.061 | 0.157 | −0.036 | 0.053 |
| 16 |
|
−0.015 | —c | −0.014 | 0.021 |
| No. | Amide | ArCHMeNHCOR | ArCHMeNHCOR |
|---|---|---|---|
a All NMR recorded at 400 MHz in CDCl3 at 10 mM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Ratio of S 1). Ratio of S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) R for 8j, 8l, 8m 1(R) R for 8j, 8l, 8m 1(R)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2(S) & for 8k 1(R) 2(S) & for 8k 1(R)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3(S). 3(S).
|
|||
| 1 | 8j |
|

|
| 2 | 8k |

|

|
| 3 | 8l |

|

|
| 4 | 8m |

|

|
The hypothesis of designing the present modification in the Kagan's reagent 1a hinges on making the aromatic ring of CSA (ArNHCO–) more electron deficient by attaching two strongly electron withdrawing groups in (S)-2 (B of Fig. 1). By making the ring electron deficient and the subsequent inductive effect (−I) we expect the hydrogen of N–H acquire more +δ character and hence becoming a better hydrogen bond donor. This hypothesis was then tested by comparing the CSA activity of Kagan's reagent 1a, the known data for 1b,19c and our present study for (S)-2 for the pivaloyl (8l) analyte derivative (Table 4). Another analogue was prepared without nitro groups (S)-7 and also scanned for the comparison. The present modified Kagan's reagent (S)-2 showed marginally better values of the nonequivalences (ΔΔδ) for the amide 8l for both the targeted hydrogens in the 1H NMR analysis supporting the concept. The other derivative (S)-7 failed to effect the discrimination in both the protons of 8l indicating the need of strongly electron withdrawing groups on the ArC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of CSA to make the carbonyl oxygen sufficiently electron deficient (+δ) for effective hydrogen bond formation. The role of hydrogen bond in the mechanism of the action of recognition of isomers for chiral solvation has been well established.29
O of CSA to make the carbonyl oxygen sufficiently electron deficient (+δ) for effective hydrogen bond formation. The role of hydrogen bond in the mechanism of the action of recognition of isomers for chiral solvation has been well established.29
Further evidence of effect of polarity of solvent was studied with conducting 1H NMR experiments in different solvents (Table 5). Very poor resolution was observed in polar solvents such as acetone-d6 and also with mixture of DMSO-d6/CDCl3 which is capable of forming hydrogen bond, while better separation was found in less polar benzene-d6.11l However, due to the high cost of benzene-d6 we have focused on the use of more commonly available chloroform-d for further study.
The two sets of signals in 1H NMR of pivaloyl derivative 8l and (S)-2 in equimolar ratio were well resolved (Fig. 3). In which both the signals CαH and the methyl signals of “S” isomer experienced up-field shift, more than the other isomer.
This separation was further studied to establish the linear relationship between the observed and actual values of ee for establishing practical utility of the CSA (Fig. 4). The observed ee values were found to be within 0.5% of actual values, which confirms the accuracy and consistency of the analysis.
 | ||
| Fig. 4 Selected region of 1H NMR spectra of scalemic mixture of 8l in presence of (S)-2 (left) and its correlation between theoretical and observed % ee values (right). | ||
The scope of the present CSA was further explored with chiral sulfoxides to determine their efficacy in recognition of the isomers. Since sulfoxides are also effective acceptors of hydrogen bonding we believe the mode of action of molecular recognition of the present CSA will be on the similar lines. Few derivatives of CSA are available to determine the enantiomer ratio by 1H NMR analysis.11g,17b,17d,30 To test the efficiency we screened a sample of unsymmetrical sulfoxides 9 with (S)-2 in CDCl3 under the established conditions (Fig. 5). A distinct shift of the signals of alkyl protons was observed with base line separation of the two sets of signals corresponding to the two enantiomers, but the values were on the lower side compared to the amide analytes 8. This could be attributed to the possibility of two point hydrogen bonding in 8 rather than one point attachment in sulfoxides 9. For comparison, our molecules showed similar values (ΔΔδ = 0.008 ppm or 3.2 Hz) compared to the 1b for separation of signals for 9a.17b
We further investigated application of (S)-2 to check separation of signals in case of racemic benzoin 10a and keto epoxide 10b a product of Darzen condensation, where the carbonyl is expected to be less polarized compared to other substrates and hence may have weaker hydrogen bonding. There are very few reports on the methods to discriminate these substrates for analytical purpose.31 Since our CSA has worked well in differentiating the isomers based on hydrogen bonding we scanned (S)-2 with racemic 10a and 10b under similar conditions and observed small shift in the signals of the CαH in 1H NMR (Fig. 5 and 6).
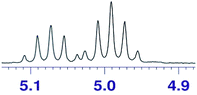
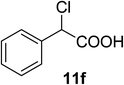
The use of chiral solvating agents (CSA) for determining optical purity by 1H NMR analysis is emerging as a useful tool and hence we need to scan different types of analytes to prove its wider applicability. Hence we further scanned the present CSA (S)-2 for α-substituted acids as they form an important class of chiral compounds. Usually the CSAs used for analyzing chiral acids are basic in nature and the mode of interactions are based on the formation of diastereomeric complex or salt with the substrates.32 The nature of (S)-2 is neutral and hence our initial experiment of mixing only mandelic acid 11a and (S)-2 in CDCl3 and recording 1H NMR did not result in any separation of signals, although a small degree of up field shift was recorded (Δδ = −0.19 ppm). The use of external base to help abstraction of acid proton of such analytes, making the hydrogen bonding possible between the carboxylate with hydrogen bond donor bis-thiourea type CSA is reported.29f For similar phenomena in the present study we initially used DMAP along with (S)-2 to determine if the CαH of mandelic acid can be distinguished in 1H NMR. Indeed, the CαH proton was observed to shift much up field and two distinct singlets were seen for the two isomers (Table 6). Further improved resolution was observed when the base was replaced with DABCO, with equimolar quantity and even better separation with excess CSA. Number of derivatives of α-hydroxy aryl acetic acids, 11b to 11e, were scanned to establish generality of the analysis. An example of α-chloro phenyl acetic 11f was also scanned with good separation. All these molecules showed a clear base line separation of the CαH signals for practical and accurate determination of optical purity.
| No. | α-Hydroxy/alkoxy acid | CSA (eq.) | Base (1 eq.) | ArCH(OH)COOH | |
|---|---|---|---|---|---|
| Δδ | ΔΔδ | ||||
a All NMR recorded at 400 MHz in CDCl3 at 10 mM (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), with indicated amount of DMAP/DABCO. 1), with indicated amount of DMAP/DABCO.
|
|||||
| 1 |

|
1.0 | DMAP | −0.278 | 0.022 |
| 2 | 11a | 1.0 | DABCO | −0.384 | 0.040 |
| 3 | 11a | 2.0 | DABCO | −0.425 | 0.047 |
| 4 |

|
2.0 | DABCO | −0.430 | 0.056 |
| 5 |

|
2.0 | DABCO | −0.402 | 0.030 |
| 6 |

|
2.0 | DABCO | −0.441 | 0.048 |
| 7 |

|
2.0 | DABCO | −0.250 | 0.066 |
| 8 |
|
2.0 | DABCO | −0.130 | 0.053 |
Natural and unnatural amino acids in optically pure form are important intermediates in the synthesis and study of bioactive molecules. We further extended the scope of our reagent (S)-2 to scan for differently N-protected phenyl glycine as a test case. The effectiveness is general for some commonly used derivatives of this amino acid (Table 7).
The importance of chiral drugs in medicinal chemistry is now a well established phenomenon. In the present work we have scanned our CSA (S)-2 for some chiral drugs and drug intermediates. Non steroidal anti-inflammatory agents such as ibuprofen 13a, ketoprofen 13b and flurbiprofen 13e are some of the important chiral drugs.34a These α-alkyl aryl acetic acids showed significant baseline splitting of signal of CαH in 1H NMR analysis (Fig. 7). The separation of signals in 19F NMR were also observed in case of 13e (Fig. 8). There is always an advantage if two nuclei can be analyzed in a chiral molecule to confirm its optical purity. Some of the optically active drugs possess sulfoxide chiral center and the activity is believed to be associated with the chirality. One of these class of drugs is used as proton pump inhibitor to treat gastroesophageal reflux disease, peptic ulcers, erosive esophagitis and Zollinger–Ellison syndrome.34b,c Omeprazole 13c is one such active drug commercially available. We scanned (S)-2 to detect discrimination of the signals in 1H NMR of 13c. However, the expected split for the α-protons of SOCH2– was not observed but the aromatic methyl signals got separated with small chemical shift nonequivalences (0.008 ppm). Another chiral molecule 2-hydroxy-3-methoxy-3,3-diphenyl propionic acid 13d, which is an intermediate for few pharmaceutical entities34d was also tested. Although CαH was not resolved completely the signals of CβOCH3 showed good separation in 1H NMR. We extend the study with the analysis of 13f, an intermediate of nebivolol, a beta blocker agent.34e In this candidate we could observe very good separation of signals of CαH in 1H NMR along with separation of 19F NMR analysis (Fig. 8).
 | ||
| Fig. 8 19F NMR spectrum of 13e (a) and 13f (b); CαH of 13f in 1H NMR, top without CSA and bottom with (S)-2 (2.0 eq.) and DABCO (1.0 eq) in CDCl3 (10 mmol) (c). | ||
Hence in this work we have developed a modified derivative of Kagan's reagent capable of distinguishing the protons of the enantiomers by simple 1H NMR experiments. We have demonstrated the improved ability of (S)-2 to accurately detect protons of a wide variety of compounds of amides, sulfoxides, benzoin, α-substituted aryl acetic acids, keto epoxides with good separations. The modified CSA has been scanned for few chiral drugs and drug intermediates with good separations. Few examples of the separation of signals in 19F NMR can widen the scope of analysis for fluorine containing chiral compounds.
Experimental section
General procedure for the enzymatic resolution
1-[3,5-bis(trifluoromethyl)phenyl] ethanol: (S)-3. M.p. = 86–87 °C (lit. (ref. 23c) 88 °C). [α]D = −24.8 (c = 1.0 CHCl3) (lit. (ref. 33) [α]D = −24.1 (c = 1.0 CHCl3)) HPLC condition for alcohol (S)-3 Chiralpak OD-H column: 5% IPA–hexane, UV = 254 nm, flow = 0.5 mL min−1 Rt – 9.5 min (1st peak)-[S-isomer] and Rt – 10.5 min (2nd peak)-[R-isomer].
1H NMR (400 MHz, CDCl3) δ 1.54 (d, J = 6.4 Hz, 3H), 2.24 (s, 1H), 5.01–5.10 (q, J = 6.4 Hz, 1H), 7.78 (s, 1H), 7.84 (s, 2H).
19F NMR (376 MHz, CDCl3): −62.88.
13C NMR (100 MHz, CDCl3): 25.5, 69.3, 121.3 (sep, JC–F = 4.4 Hz), 123.3 (q, JC–F = 271.0 Hz), 125.6, 131.9 (q, JC–F = 33.0 Hz), 148.2.
Mass (EI): 258(10), 242(100), 243(96), 240(20), 195(74), 194(87), 69(50).
IR (KBr): ν 3257–3159, 2980, 1625, 1467, 1280, 1024, 924, 896, 842, 705, 683 cm−1.
Procedure for enzymatic reaction followed by Mitsunobu reaction. After enzymatic resolution catalyst was filtered off. To the filtrate AcOH (0.11 mL, 1.93 mmol) and triphenyl phosphine (0.51 g, 1.93 mmol) were added under nitrogen atmosphere followed by the slow addition of solution of DEAD (0.38 mL, 2.42 mmol) in dry THF (1 mL) at 0 °C. The reaction mixture was stirred (6 h). The solvent was removed under reduced pressure and the crude product was purified by silica-gel column chromatography with ethyl acetate and petroleum ether (2%).
White solid 0.82 g (70.7% yield) m.p. = 59–60 °C [α]D = +55.2 (c = 1.0 methanol) lit. (ref. 23c) [α]D = +57 (c = 1.0 methanol) for (R)-isomer.
1H NMR (400 MHz, CDCl3): δ 1.59 (d, J = 6.8 Hz, 3H), 2.24 (s, 3H), 5.94–5.99 (q, J = 6.8 Hz, 1H), 7.81 (s, 1H), 7.83 (s, 2H).
19F NMR (376 MHz, CDCl3): δ −62.90.
13C NMR (100 MHz, CDCl3): δ 21.1, 22.3, 70.9, 121.9 (sep, JC–F = 4.4 Hz), 123.2, (q, JC–F = 271 Hz), 126.2, 131.4 (q, JC–F = 33 Hz), 144.3, 170.1.
Mass (EI): 299(18), 281(10), 258(100), 257(89), 239(91), 238(56), 200(47), 150(21), 71(18).
IR (KBr): ν 3007, 2941.61, 1732, 1456, 1285, 1173, 1021, 898, 706, 684 cm−1.
General procedure for hydrolysis of (R)-4
To a solution of (R)-4 (0.82 g, 3.18 mmol) in methanol (15 mL), HCl (0.5 mL, 4.76 mmol, 36%) was added. The reaction mixture was refluxed (3 h). After completion of reaction, as indicated by TLC, MeOH was evaporated under reduce pressure. The residue was taken in ethyl acetate, and washed with water. The organic layer was dried with sodium sulphate and concentrated to afford (R)-3 in 0.80 g (98%).1H NMR (400 MHz, CDCl3) δ 1.56 (d, J = 7.2 Hz, 3H), 5.79–5.86 (q, J = 7.2 Hz, 1H), 6.16 (d, J = 7.2 Hz, 1H), 7.28–7.23 (m, 2H), 7.40–7.36 (m, 1H), 7.47–7.51 (m, 2H), 7.74–7.78 (m, 2H), 8.04 (s, 2H).
19F NMR (376 MHz, CDCl3): δ −62.76.
13C NMR (100 MHz, CDCl3): δ 17.4, 48.7, 121.9 (sep, JC–F = 4.4 Hz), 123.2 (q, JC–F = 271 Hz), 127.9, 131.6, 131.7, 132.3 (q, JC–F = 33 Hz), 134.3, 142.6, 167.8.
Mass (EI): 387(89), 386(53), 372(100), 371(84), 368(89), 343(42), 239(25), 159(45).
IR (KBr): ν 2999, 2923, 1780, 1705, 1467, 1284, 1126, 1060, 895, 712, 528 cm−1.
HRMS (TOF-MS) m/z calculated for C18H11F6NO2 [M + H]+ 388.0772, found 388.0773.
General procedure for synthesis of amide ligand
1H NMR (400 MHz, CDCl3): δ 1.75 (d, J = 7.2 Hz, 3H), 5.43–5.50 (m, 1H), 6.90 (d, J = 6.8 Hz, 1H), 7.85 (s, 1H), 7.87 (s, 2H), 9.00 (d, J = 2.0 Hz, 2H), 9.20–9.211 (t, J = 2.0 Hz, 1H).
19F NMR (376 MHz, CDCl3): δ −62.78.
13C NMR (100 MHz, CDCl3): δ 21.6, 49.8, 121.5, 121.9 (sep JC–F = 4.4 Hz), 127.3, 125.8 (q, JC–F = 271 Hz), 126.6, 132.3 (q, JC–F = 33 Hz), 136.9, 144.8, 148.7, 162.1.
Mass (EI): δ 451(29), 450(11), 240(44), 195(100).
IR (KBr): ν 3343, 3088, 1647, 1544, 1348, 1278, 897, 731 cm−1.
HRMS (TOF-MS) m/z calculated for C17H11F6N3O5 [M − H]− 450.0530, found 450.0527.
1H NMR (400 MHz, CDCl3) δ 1.66 (d, J = 7.2 Hz, 3H), 5.38–5.45 (m, 1H), 6.44 (d, J = 6.8 Hz), 7.46–7.58 (m, 3H), 7.79–7.78 (m, 3H), 7.84 (s, 2H).
19F NMR (376 MHz, CDCl3): δ −62.77.
13C NMR (100 MHz, CDCl3): δ 21.9, 48.9, 121.4 (sep JC–F = 4.4 Hz), 123.3 (q, JC–F = 271 Hz), 126.4, 126.9, 128.7, 131.9, 132.4 (q, JC–F = 33 Hz), 133.7, 146.2, 166.9.
Mass (EI): δ 361(100), 360(58), 240(19), 105(90), 104(71).
IR (KBr): ν 3340, 3087, 1639, 1530, 1382, 1124, 920, 895, 845, 703 cm−1.
HRMS (ESI+) m/z calculated for C17H13F6NO [M + Na]+ 384.0799, found 384.0794.
Acknowledgements
We thank Council of Scientific and Industrial Research (CSIR), New Delhi for the award of Senior Research Fellowship to one of us (NJ). We are also grateful to DST-PURSE for the single crystal X-ray diffraction facility of the Faculty of Science. We thank Novozyme, Bangalore (India) for the generous gift of immobilized enzyme and Navin Fluorine International Limited, Surat (India) for the gift of 3,5-bis(trifluoromethyl)acetophenone.References and Notes
- (a) W. H. Pirkle and Y. Liu, J. Chromatogr. A, 1996, 736, 31 CrossRef CAS; (b) X. Gao and H. B. Kagan, Chirality, 1998, 10, 120 CrossRef CAS; (c) C. Gennary, S. Ceccarelli, U. Piarulli, C. A. G. N. Montalbetti and R. F. W. Jackson, J. Org. Chem., 1998, 63, 5312 CrossRef; (d) E. Yashima, J. Chromatogr. A, 2001, 906, 105 CrossRef CAS PubMed; (e) C. Wolf and P. A. Hawes, J. Org. Chem., 2002, 67, 2727 CrossRef CAS PubMed; (f) A. Duursma, A. J. Minnaard and B. L. Feringa, Tetrahedron, 2002, 58, 5773 CrossRef CAS; (g) X. Lai and S.-C. Ng, Tetrahedron Lett., 2003, 44, 2657 CrossRef CAS; (h) C. Wolf, Z. Fadul, P. A. Hawes and E. C. Volpe, Tetrahedron: Asymmetry, 2004, 15, 1987 CrossRef CAS; (i) C. Moiteiro, N. Fonseca, M. J. M. Curto, R. Tavares, A. M. Lobo, P. Ribeiro-Claro, V. Félix and M. G. B. Drew, Tetrahedron: Asymmetry, 2006, 17, 3248 CrossRef CAS.
- (a) J. Guo, J. Wu, G. Siuzdak and M. G. Finn, Angew. Chem., Int. Ed., 1999, 38, 1755 CrossRef CAS; (b) M. T. Reetz, M. H. Becker, H.-W. Klein and D. Stockigt, Angew. Chem., Int. Ed., 1999, 38, 1758 CrossRef CAS; (c) C. Markert and A. Pfaltz, Angew. Chem., Int. Ed., 2004, 43, 2498 CrossRef CAS PubMed.
- (a) M. T. Reetz, M. H. Becker, K. M. Kuhling and A. Holzwarth, Angew. Chem., Int. Ed., 1998, 37, 2647 CrossRef CAS; (b) R. Eelkema, R. A. van Delden and B. L. Feringa, Angew. Chem., Int. Ed., 2004, 43, 5013 CrossRef CAS PubMed; (c) L. Zhu and E. V. Anslyn, J. Am. Chem. Soc., 2004, 126, 3676 CrossRef CAS PubMed; (d) X. Mei and C. Wolf, Chem. Commun., 2004, 2078 RSC; (e) J. F. Folmer-Andersen, V. M. Lynch and E. V. Anslyn, J. Am. Chem. Soc., 2005, 127, 7986 CrossRef CAS PubMed; (f) G. E. Tumambac and C. Wolf, Org. Lett., 2005, 7, 4045 CrossRef CAS PubMed; (g) X. Mei and C. Wolf, J. Am. Chem. Soc., 2006, 128, 13326 CrossRef CAS PubMed; (h) D. Leung and E. V. Anslyn, J. Am. Chem. Soc., 2008, 130, 12328 CrossRef CAS PubMed.
- (a) K. Ding, A. Shii and K. Mikami, Angew. Chem., Int. Ed., 1999, 38, 497 CrossRef CAS; (b) M. T. Reetz, D. Kuhling, H. Hinrichs and D. Belder, Angew. Chem., Int. Ed., 2000, 39, 3891 CrossRef CAS; (c) S. Nieto, J. M. Dragna and E. V. Anslyn, Chem.–Eur. J., 2010, 16, 227 CrossRef CAS PubMed; (d) M. W. Ghosn and C. Wolf, J. Am. Chem. Soc., 2009, 131, 16360 CrossRef CAS PubMed.
- (a) P. Abato and C. T. Seto, J. Am. Chem. Soc., 2001, 123, 9206 CrossRef CAS PubMed; (b) M. Matsushita, K. Yoshida, N. Yamamoto, P. Wirsching, R. A. Lerner and K. D. Janda, Angew. Chem., Int. Ed., 2003, 42, 5984 CrossRef CAS PubMed.
- (a) D. Parker, Chem. Rev., 1991, 91, 1441 CrossRef CAS; (b) J. M. Seco, E. Quiñoá and R. Riguera, Chem. Rev., 2004, 104, 17 CrossRef CAS; (c) J. Labuta, S. Ishihara, T. Šikorský, Z. Futera, A. Shundo, L. Hanyková, J. V. Burda, K. Ariga and J. P. Hill, Nat. Commun., 2013, 4, 1 Search PubMed.
- (a) W. H. Pirkle and S. D. Beare, J. Am. Chem. Soc., 1969, 91, 5150 CrossRef CAS; (b) W. H. Pirkle, R. L. Muntz and I. C. Paul, J. Am. Chem. Soc., 1971, 93, 2817 CrossRef CAS.
- (a) B. C. Mayo, J. Am. Chem. Soc., 1969, 91, 5160 CrossRef; (b) J. H. Liu and J. T. Tsay, Analyst, 1982, 107, 544 RSC; (c) M. D. McCreary, D. W. Lewis, D. L. Wernick and G. M. Whitesides, J. Am. Chem. Soc., 1974, 96, 1038 CrossRef CAS; (d) H. L. Goering, J. N. Eikenberry, G. S. Koermer and C. J. Lattimer, J. Am. Chem. Soc., 1974, 96, 1493 CrossRef CAS; (e) T. J. Wenzel and J. D. Wilcox, Chirality, 2003, 15, 256 CrossRef CAS PubMed; (f) V. E. U. Costa and J. E. D. Martins, Ann. Magn. Reson., 2006, 5, 5 Search PubMed; (g) Dynamic stereochemistry of chiral compounds, ed. C. Wolf, RSC, Cambridge, 2008, pp. 143–145 Search PubMed.
- (a) J. Jacobus, M. Raban and K. Mislow, J. Org. Chem., 1968, 33, 1142 CrossRef CAS; (b) J. A. Dale, D. L. Dull and H. S. Mosher, J. Org. Chem., 1969, 34, 2543 CrossRef CAS; (c) R. C. Anderson and M. J. Shapiro, J. Org. Chem., 1984, 49, 1304 CrossRef CAS; (d) T. K. Yahuuchi, J. Org. Chem., 2000, 65, 397 CrossRef; (e) A. Alexakis and A.-S. Chauvin, Tetrahedron: Asymmetry, 2001, 12, 1411 CrossRef CAS; (f) K. Blazewska and T. Gajda, Tetrahedron: Asymmetry, 2002, 13, 671 CrossRef CAS; (g) S. Rodriguez-Escrich, D. Popa, C. Jimeno, A. Vidal-Ferran and M. A. Pericas, Org. Lett., 2005, 7, 3829 CrossRef CAS PubMed; (h) T. Reiner, F. N. Naraschewski and J. Eppinger, Tetrahedron: Asymmetry, 2009, 20, 362 CrossRef CAS; (i) T. J. Wenzel and C. D. Chisholm, Chirality, 2011, 23, 190 CrossRef CAS PubMed.
- (a) S. Porto, J. M. Seco, J. F. Espinosa, E. Quiñoá and R. Riguera, J. Org. Chem., 2008, 73, 5714 CrossRef CAS PubMed; (b) N. V. Orlov and V. P. Ananikov, Green Chem., 2011, 13, 1735 RSC.
- (a) W. H. Pirkle, J. Am. Chem. Soc., 1966, 88, 1837 CrossRef CAS; (b) T. G. Burlingame and W. H. Pirkle, J. Am. Chem. Soc., 1966, 88, 5150 CrossRef; (c) T. J. Wenzel, E. P. Amanoo, S. S. Shariff and S. E. Aniagyei, Tetrahedron: Asymmetry, 2003, 14, 3099 CrossRef CAS; (d) X. Yang, G. Wang, C. Zhong, X. Wu and E. Fu, Tetrahedron: Asymmetry, 2006, 17, 916 CrossRef CAS; (e) A. E. Lovelym and T. J. Wenzel, J. Org. Chem., 2006, 71, 9178 CrossRef PubMed; (f) M. Palomino-Schätzlein, A. Virgili, S. Gil and C. Jaime, J. Org. Chem., 2006, 71, 8114 CrossRef PubMed; (g) T. Ema, D. Tanida and T. Sakai, J. Am. Chem. Soc., 2007, 129, 10591 CrossRef CAS PubMed; (h) M. Ma, L. Ai, X. Shen and C. Zhang, Org. Lett., 2007, 9, 125 CrossRef PubMed; (i) F. Ma, X. Shen, X. Ming, J. Wang, J. Ou-Yang and C. Zhang, Tetrahedron: Asymmetry, 2008, 19, 1576 CrossRef CAS; (j) A. Gualandi, S. Grilli, D. Savoia, M. Kwit and J. Gwaroński, Org. Biomol. Chem., 2011, 9, 4234 RSC; (k) D. Kumari, P. Bandyopadhyay and N. Suryaprakash, J. Org. Chem., 2013, 78, 2373 CrossRef CAS PubMed; (l) I. Pal, S. R. Chaudhari and N. Suryaprakash, New J. Chem., 2014, 38, 4908 RSC; (m) K. Tanaka, T. Iwashita, C. Sasaki and H. Takahashi, Tetrahedron: Asymmetry, 2014, 25, 602 CrossRef CAS.
- 13C NMR CSA: M. Pérez-Trujillo, E. Monteagudo and T. Parella, Anal. Chem., 2013, 85, 10887 CrossRef PubMed.
- 19F NMR CSA: (a) S. Rodríguez-Escrich, D. Popa, C. Jimeno, A. Vidal-Ferran and M. A. Pericàs, Org. Lett., 2005, 7, 3829 CrossRef PubMed; (b) S. R. Chaudhari and N. Suryaprakash, Org. Biomol. Chem., 2012, 10, 6410 RSC.
- 31P NMR CSA: (a) X. Liu, P. Ilankumaran, I. A. Guzei and J. G. Verkade, J. Org. Chem., 2000, 65, 701 CrossRef CAS; (b) J. Bravo, C. Cativiela, J. E. Chaves, R. Navarro and E. P. Urriolabeitia, Inorg. Chem., 2003, 42, 1006 CrossRef CAS PubMed; (c) D. Magiera, J. Omelanczuk, K. Dziuba, K. M. Pietrusiewicz and H. Duddeck, Organometallics, 2003, 22, 2464 CrossRef CAS; (d) F. Ma, X. Shen, J. Ou-Yang, Z. Deng and C. Zhang, Tetrahedron: Asymmetry, 2008, 19, 31 CrossRef CAS; (e) O. S. Fenton and B. R. Sculimbrene, J. Chem. Educ., 2011, 88, 662 CrossRef CAS.
- 77Se NMR CSA: (a) P. H. Menezes, S. M. C. Gonçalves, F. Hallwass, R. O. Silva, L. W. Bieber and A. M. Simas, Org. Lett., 2003, 5, 1601 CrossRef CAS PubMed; (b) N. V. Orlov and V. P. Ananikov, Chem. Commun., 2010, 46, 3212 RSC; (c) J. G. Ferreira and S. M. C. Gonçalves, J. Braz. Chem. Soc., 2010, 21, 2023 CrossRef CAS.
- 195Pt NMR CSA: G. Uccello-Barretta, R. Bernardini, F. Balzano and P. Salvadori, Chirality, 2002, 14, 484 CrossRef CAS PubMed.
- (a) P. Pitchen, E. Duñach, M. Deshmukh and H. B. Kagan, J. Am. Chem. Soc., 1984, 106, 8188 CrossRef CAS; (b) M. Deshmukh, E. Duñach, S. Juge and H. B. Kagan, Tetrahedron Lett., 1984, 25, 3467 CrossRef CAS; (c) E. Duñach and H. B. Kagan, Tetrahedron Lett., 1985, 26, 2649 CrossRef; (d) P. Chaprin, E. Duñach, H. B. Kagan and F. R. Theobald, Tetrahedron Lett., 1986, 27, 2989 CrossRef.
- (a) W. H. Pirkle, P. L. Spence and B. Lamm, J. Org. Chem., 1992, 57, 3854 CrossRef CAS; (b) W. H. Pirkle and C. J. Welch, J. Liq. Chromatogr., 1992, 15, 1947 CrossRef CAS; (c) W. H. Pirkle, L. J. Brice and G. J. Terfloth, J. Chromatogr. A, 1996, 753, 109 CrossRef CAS.
- (a) Z. Pakulski, O. M. Demchuk, R. Kwiatosz, P. W. Osiński, W. Świerczyńska and K. M. Pietrusiewicz, Tetrahedron: Asymmetry, 2003, 14, 1459 CrossRef CAS; (b) M. E. Koscho, P. L. Spence and W. H. Pirkle, Tetrahedron: Asymmetry, 2005, 16, 3147 CrossRef CAS; (c) D. P. Iwaniuk and C. Wolf, J. Org. Chem., 2010, 75, 6724 CrossRef CAS PubMed; (d) C. Wolf, A. M. Cook and J. E. Dannatt, Tetrahedron: Asymmetry, 2014, 25, 163 CrossRef CAS.
- A. Bilz, T. Stork and G. Helmchen, Tetrahedron: Asymmetry, 1997, 8, 3999 CrossRef CAS.
- K. Faber, Biotransformations in organic chemistry, Springer-Verlag, Berlin, Heidelberg, 6th edn, 2011 Search PubMed.
- (a) N. Jain and A. V. Bedekar, Tetrahedron: Asymmetry, 2011, 22, 1176 CrossRef CAS; (b) N. Jain, M. B. Mandal and A. V. Bedekar, Tetrahedron, 2014, 70, 4343 CrossRef CAS.
- (a) T. Matsuda, K. Tsuji, T. Kamitanaka, T. Harada, K. Nakamura and T. Ikaria, Chem. Lett., 2005, 34, 1102 CrossRef CAS; (b) K. Bogár and J.-E. Bäckvall, Tetrahedron Lett., 2007, 48, 5471 CrossRef; (c) P. J. Vankawala, N. Kolla, C. R. Elati, M. Sreenivasulu, A. K. Kumar, Y. Anjaneyulu, S. Venkatraman, A. Bhattacharya and V. T. Mathad, Synth. Commun., 2007, 37, 3439 CrossRef CAS.
- (a) O. Mitsunobu and Y. Yamada, Bull. Chem. Soc. Jpn., 1967, 40, 2380 CrossRef CAS; (b) K. C. Swamy, N. N. Kumar, E. Balaraman and K. V. Kumar, Chem. Rev., 2009, 109, 2551 CrossRef CAS PubMed.
- (a) E. Vänttinen and L. T. Kanerva, Tetrahedron: Asymmetry, 1995, 6, 1779 CrossRef; (b) N. Bouzemi, L. Aribi-Zouioueche and J.-C. Fiaud, Tetrahedron: Asymmetry, 2006, 17, 797 CrossRef CAS; (c) Y. Shimotori and T. Miyakoshi, Synth. Commun., 2010, 40, 1607 CrossRef CAS; (d) T. Das, R. Bhuniya and S. Nanda, Tetrahedron: Asymmetry, 2010, 21, 2206 CrossRef CAS; (e) Z. Houiene, M. Merabet-Khelassi, N. Bouzemi, O. Riant and L. Aribi-Zouioueche, Tetrahedron: Asymmetry, 2013, 24, 290 CrossRef CAS.
- J. Mangas-Sánchez, M. Rodríguez-Mata, E. Busto, V. Gotor-Fernández and V. Gotor, J. Org. Chem., 2009, 74, 5310 CrossRef PubMed.
- M. Kanai, M. Yasumoto, Y. Kuriyama, K. Inomiya, Y. Katsuhara, K. Higashiyama and A. Ishii, Org. Lett., 2003, 5, 1007 CrossRef CAS PubMed.
- ESI†.
- (a) B. C. Hamann, N. R. Branda and J. Rebek Jr, Tetrahedron Lett., 1993, 34, 6837 CrossRef CAS; (b) G. M. Kyne, M. E. Light, M. B. Hursthouse, J. D. Mendoza and J. D. Kilburn, J. Chem. Soc., Perkin Trans. 1, 2001, 1258 RSC; (c) J. Wagger, S. G. Grdadolnik, U. Grošelj, A. Meden, B. Stanovnik and J. Svete, Tetrahedron: Asymmetry, 2007, 18, 464 CrossRef CAS; (d) M. Hernandez-Rodriguez and E. Juaristi, Tetrahedron, 2007, 63, 7673 CrossRef CAS; (e) S. R. Chaudhari and N. Suryaprakash, J. Mol. Struct., 2012, 1016, 163 CrossRef CAS; (f) G. Bian, H. Fan, S. Yang, H. Yue, H. Huang, H. Zong and L. Song, J. Org. Chem., 2013, 78, 9137 CrossRef CAS PubMed; (g) S. R. Chaudhari and N. Suryaprakash, New J. Chem., 2013, 37, 4025 RSC.
- (a) T. J. Wenzel and K. L. Brogan, Enantiomer, 2000, 5, 293 CAS; (b) C. Meyer and H. Duddeck, Magn. Reson. Chem., 2000, 38, 29 CrossRef CAS; (c) A. E. Tremblay, N. Tan, E. Whittle, D. J. Hodgson, B. Dawson, P. H. Buist and J. Shanklin, Org. Biomol. Chem., 2010, 8, 1322 RSC; (d) C. Zonta, A. Kolarovic, M. Mba, M. Pontini, E. P. Kündig and G. Licini, Chirality, 2011, 23, 796 CrossRef CAS PubMed.
- T. Ema, D. Tanida, K. Sugita, T. Sakai, K.-I. Miyazawa and A. Ohnishi, Org. Lett., 2008, 10, 2365 CrossRef CAS PubMed.
- A. R. Chaudhary, P. Yadav and A. V. Bedekar, Tetrahedron: Asymmetry, 2014, 25, 767 CrossRef CAS and the references cited therein.
- J.-H. Xie, X.-Y. Liu, J.-B. Xie, L.-X. Wang and Q.-L. Zhou, Angew. Chem., Int. Ed., 2011, 50, 7329 CrossRef CAS PubMed.
- (a) R. Fulwood and D. Parker, J. Chem. Soc., Perkin Trans. 2, 1994, 57 RSC; (b) P. Lindberg, A. Brändström, B. Wallmark, H. Mattsson, L. Rikner and K.-L. Hoffman, Med. Res. Rev., 1990, 10, 1 CrossRef CAS PubMed; (c) H. Cotton, T. Elebring, M. Larsson, L. Li, H. Sörensen and S. von Unge, Tetrahedron: Asymmetry, 2000, 11, 3819 CrossRef CAS; (d) W. Amberg, S. Hergenröder, H. Hillen, J. Jansen, G. Kettschau, A. Kling, D. Klinge, M. Raschack, H. Riechers and L. Unger, J. Med. Chem., 1999, 42, 3026 CrossRef CAS PubMed; (e) A. Veverka, D. S. Nuzum and J. L. Jolly, Ann. Pharmacother., 2006, 40, 1353 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1042105. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra06959a |
| This journal is © The Royal Society of Chemistry 2015 |