Mechanochemical growth of a porous ZnFe2O4 nano-flake thin film as an electrode for supercapacitor application
M. M. Vadiyara,
S. C. Bhisea,
S. K. Patila,
S. A. Patila,
D. K. Pawara,
A. V. Ghule*b,
P. S. Patilc and
S. S. Kolekar*a
aAnalytical Chemistry and Material Science Research Laboratory, Department of Chemistry, Shivaji University, Kolhapur 416004, Maharashtra, India. E-mail: sskolekar@gmail.com
bGreen Nanotechnology Laboratory, Department of Chemistry, Shivaji University, Kolhapur 416004, Maharashtra, India. E-mail: anighule@gmail.com
cThin Film Materials Laboratory, Department of Physics, Shivaji University, Kolhapur 416 004, India
First published on 15th May 2015
Abstract
Herein, we are reporting a simple, economic, easy to handle, scalable and reproducible mechanochemical i.e. rotational chemical bath deposition (R-CBD) approach for the synthesis of well adhered nano-flake ZnFe2O4 thin films (NFs-ZnFe2O4) with uniform morphology on a stainless steel (SS) substrate, in comparison with nano-grain ZnFe2O4 thin films (NGs-ZnFe2O4) prepared using a conventional CBD approach. The influence of rotation on the evolution of the nano-flake morphology in NFs-ZnFe2O4 is also investigated. The porous NFs-ZnFe2O4 thin films demonstrated excellent pseudocapacitor properties with higher specific capacitance of 768 F g−1 at high current density of 5 mA cm−2, stability upto 5000 cycles (88% retention), higher energy density (106 W h kg−1) and power density (18 kW kg−1) compared to NGs-ZnFe2O4. The results were also found to be higher than those reported earlier for MFe2O4 based systems.
1 Introduction
Developing supercapacitor (SC) energy storage devices with high power density and excellent cycle stability that can fulfil the continuously growing energy demand while addressing environmental concerns have attracted the potential interest of the scientific community.1,2 Supercapacitors are classified into electric double layer capacitors (EDLCs, energy is stored by charge accumulation) and pseudocapacitors (energy stored by fast reversible faradic reactions on the surface of electrode materials) based on their energy storage mechanism.3,4 Among the several materials explored, transition metal oxides (TMOs)5,6 such as MCo2O4 (M = Ni, Zn, Mg, Cu, Mn) MFe2O4 (M = Co, Ni, Cu and Zn)7–11 remain the most explored candidates as pseudocapacitive electrodes for SCs to improve the energy density and overall performance. Ferrites including magnetite of Fe3O4, CoFe2O4, MnFe2O4, and CuFe2O4 (ref. 9, 11–14) and their nanocomposites with conducting additives which could produce higher specific capacitance than typical carbonaceous materials with electric double layer capacitance have also been explored with great interest (Table 1).15–17| Sr. no. | Method | Nature of ferrite films | Structure | Current collector | Electrolyte with molar concentration | Maximum capacitance F g−1 | Current density mA cm−2 or scan rate mV s−1 | References (year) |
|---|---|---|---|---|---|---|---|---|
| 1 | Precipitation | MnFe2O4 powder | Granular | Platinum mesh | 1 M NaCl | 100 F g−1 | 10 mV s−1 | (2005)12 |
| 2 | Hydrothermal | NiFe2O4/graphene powder | Particles | Platinum electrode | 1 M Na2SO4 | 345 F g−1 | 1 A g−1 | (2013)34 |
| 3 | Chemical bath deposition | CoFe2O4 thin film | Nano-flakes | Graphite | 1 M Na2SO4 | 366 F g−1 | 10 mV s−1 | (2012)9 |
| 4 | Solvothermal | CuFe2O4 powder | Nanospheres | Platinum wire | 1 M KOH | 334 F g−1 | 0.6 A g−1 | (2013)13 |
| 5 | Hydrothermal | CoFe2O4 thin film | Nano-flowers | Platinum plate | 1 M KOH | 768 F g−1 | 6 A g−1 | (2015)36 |
| 6 | Chemical bath deposition | ZnFe2O4 thin film | Nano-flakes | Graphite | 3 M KOH | 768 F g−1 | 5 mA cm−2 | Present work |
| Nano-grains | 534 F g−1 |
Recently, spinel transition metal oxides (AB2O4) have attracted great attention as promising electrode material with the use of two metal elements. Specifically, nickel cobaltite (NiCo2O4) and zinc cobaltite (ZnCo2O4) are the most studied compositions because of their higher electrochemical activity, low cost, abundant resources and environmental friendliness when compared with monometallic counterparts such as nickel oxide or cobalt oxide.18,19 Moreover, the potential window for NiCo2O4 is only 0.4–0.6 V,17 which hardly finds practical applications. Kumbhar and coworkers reported spinel CoFe2O4 thin film prepared by using chemical bath deposition which demonstrated specific capacitance of 366 F g−1 at a constant and high potential window of 1.0 V.9,17 Particularly, among the spinel metal ferrites (MFe2O4) system, ZnFe2O4 is promising due to its low toxicity, high specific surface area, highly electroactive nature, good chemical and thermal stability, environmental benignity, low cost and rich abundance on Earth. Meanwhile, developing porous nanostructured thin films for SCs hold great promise considering its porosity and high surface area, which contributes in enhancement of the performance by facilitating ion transport and creating more-active reaction sites.14 With this motivation, we report for first time a facile approach of selectively developing interconnected porous nano-flakes ZnFe2O4 (NFs-ZnFe2O4) thin films using rotational chemical bath deposition (R-CBD) and comparing with the ZnFe2O4 thin films prepared using conventional CBD having nano-grain morphology (NGs-ZnFe2O4).
2 Experimental
2.1 Chemicals
Unless until specified, all the chemical and reagents used in this work are AR grade chemicals. Zinc chloride (ZnCl2), ferrous chloride tetrahydrate (FeCl2·4H2O), mono-ethanolamine (MEA) and ammonia received from (Merck chemicals) were used as such for the thin film deposition.2.2 Synthesis of NFs-ZnFe2O4 and NGs-ZnFe2O4 thin films
The ZnFe2O4 thin films were prepared by heating mixture of aqueous alkaline solutions of ZnCl2 (0.1 M, 25 mL) and FeCl2·4H2O (0.2 M, 25 mL) in a water bath maintained at 55 °C. The mixture of aqueous solution was first stirred for 15 min to ensure complete dissolution resulting in clear solution. This was followed by addition of 3 mL of mono-ethanolamine (MEA) as a complexing agent and dropwise addition of ammonia for adjusting the pH 10 (±0.5) to form blue coloured solution. Further, ultrasonically cleaned stainless steel substrates were immersed into the bath solution when the temperature reached 35 °C and subsequently the temperature was raised to 55 °C. In the first case, the deposition was achieved without rotation of the stainless substrate immersed in solution bath and is referred as conventional CBD (C-CBD). In the second case, the stainless steel substrate immersed in solution bath was rotated using gear motor with speed controller during the deposition of the thin film (R-CBD). The ZnFe2O4 thin films obtained by R-CBD were studied for their morphological changes at different rotation speeds of 15, 30, 45, 55 and 65 rpm. The heterogeneous reactions that initiate on the substrates involve the adsorption of atoms when the solution reaches the super-saturation. In both the cases, the deposition was carried out for 3 h to ensure completion of reaction. Increase in thicknesses of the ZnFe2O4 thin films was noted with increasing deposition time. The ZnFe2O4 thin film deposited substrates were further removed from the bath and washed thoroughly with double distilled water and subsequently by ethanol. The thin films were further dried in oven at 60 °C for 5 h in air atmosphere and later annealed at 550 °C to ensure decomposition of organic matter and transformation of hydroxide phase into crystalline phase.202.3 Material characterizations
Both NFs-ZnFe2O4 and NGs-ZnFe2O4 thin film samples were characterized for their structure, composition, thermal stability, morphology and supercapacitive properties. The crystalline quality, phase identification are characterized by XRD (Bruker, D2-Phaser X-ray diffractometer) with CuKα1 (λ = 1.5406 Å) radiation in the range 20–80°. The TGA thermograms were obtained using TG Analyzer (SDT Q600 V20.9 Build 20) in the temperature range from 25 to 800 °C, with heating rate 10 °C min−1 in air atmosphere. Raman spectra were obtained using Horiba Jobin Yvon with Lab RAM HR system at room temperature using a 532 nm solid state laser as excitation source. The surface morphology of the ZnFe2O4 thin films was characterized by scanning electron microscopy (SEM) using JSM-6360 JEOL SEM and Hitachi, S-4700. X-ray photoelectron spectroscopy (XPS-PHI 5300 PHI USA) was used to study the chemical composition and oxidation state. BET surface area analysis and pore size analysis was performed using Quantachrome NOVA1000e, USA.2.4 Electrochemical characterization
The NFs-ZnFe2O4 and NGs-ZnFe2O4 thin film samples were explored for supercapacitor application by constructing the cell involving three electrode system. The cyclic voltammetry (CV) curves were obtained at different scan rates of 10 to 100 mV s−1. Charge–discharge study as function of varying current densities from 5 to 30 mA cm−2 and electrochemical impedance spectroscopy (EIS) in the frequency range from 1 Hz to 100 kHz was performed using CHI 608E electrochemical analyzer.2.5 Construction of a supercapacitor cell
The electrochemical cell with three electrode system was composed of graphite (G) as a current collector, silver–silver chloride (Ag/AgCl) as reference electrode and prepared NFs-ZnFe2O4/NGs-ZnFe2O4 thin film on stainless steel (SS) substrate as working electrode, while aqueous 3 M KOH solution was used as electrolyte as shown in following eqn (1) and (2).21| SS/NFs-ZnFe2O4/3 M KOH (aq)/Ag/AgCl/G | (1) |
| SS/NGs-ZnFe2O4/3 M KOH (aq)/Ag/AgCl/G | (2) |
3 Results and discussion
3.1 Formation mechanism and reaction of NFs-ZnFe2O4 and NGs-ZnFe2O4 thin films
The fabrication process of the NFs-ZnFe2O4 and NGs-ZnFe2O4 thin films are shown in Scheme 1. First, NFs-ZnFe2O4 thin films were grown on stainless steel substrates via R-CBD based on the mechanical clockwise rotation of the substrate at varying rotations, however, sample prepared using 55 rpm is taken as representative sample. On the other hand, NGs-ZnFe2O4 thin films were obtained using conventional CBD in which substrate was fixed (0 rpm). The rotation of substrate enhances the mobility of molecules due to collision, probability of interaction and reaction; hence it increases the number nucleation sites with fine particle size. The fast dissociation rate of mono-ethanolamine (MEA) determines the kinetics of the reaction between dissociated OH and corresponding metal precursors. In comparison, the sample with the fixed substrate (0 rpm) produces nano-grains morphology as a result of slow movement of atoms decreasing the rate of dissociation of mono-ethanolamine (MEA) and metal precursors, followed by aggregation.22The deposition of ZnFe2O4 thin films follows three step reactions. First step involves the nucleation process in which complexation of Zn2+ and Fe2+ with complexing agent MEA takes place. The second step involves super-saturation of the solution because of increased ratio of ionic product and solubility product, which influences the growth of nuclei and thickness of the film. The third step is the termination step of the reaction which retards the growth of the film. The overall reaction is presented in the reaction mechanism 1. The reactions (1) and (2) depict the formation of metal hydroxides from the corresponding metal complexes. The reaction (3) shows the oxidation of ferrous hydroxide to goethite (iron oxyhydroxide). The fourth reaction presents the reaction of zinc hydroxide and goethite to form crystalline zinc ferrite thin films on the SS substrates by removing water while annealing temperature of 550 °C is set.22
3.2 Morphological, thermal, structural, surface area and elemental analysis of ZnFe2O4 thin films
In order to clarify the formation mechanism of the NFs-ZnFe2O4 and NGs-ZnFe2O4 thin films on stainless steel, the products grown at various rotations speed were investigated by SEM.Initially the substrates are fixed (0 rpm) in the solution which gives nano-grain like morphology as clearly observed on the surface of stainless steel (Fig. 1(a)). When the rotation was applied to substrates, as a result of the mechanical force the nano-grains were found to be transformed into interconnected nano-flakes like morphology. The initial rotation speed of the 15 rpm shows mixed growth of nano-flakes and nano-grains (Fig. 1(b)) which confirms the transformation of nano-grain structure into nano-flakes. When the rotation speed increased to 30 rpm the shape and size of nano-grains are partially converted into uniformly adhered nano-flakes (Fig. 1(c)). The rotation speed was further extended to 45 rpm which shows well defined porous networks of the nano-flakes as shown in Fig. 1(d). For the further confirmation, the rotation speed was increased to 55 and 65 rpm (Fig. 1(e) and (f)) which showed nearly same morphology and demonstrated the growth of uniform size (∼25 nm) nano-flakes. NFs-ZnFe2O4 film did not detach from the stainless steel, even after higher rotation speeds, suggesting that the film was strongly adhered to the substrate. This strong adhesion was presumably due to the maximum reaction sites obtained on the conducting substrates by rotation. Among the different rotation speeds we use 0 rpm and 55 rpm for the further characterization and electrochemical measurement study.
 | ||
| Fig. 1 SEM images obtained from different rotation speeds (a) 0 rpm (b) 15 rpm (c) 30 rpm (d) 45 rpm (e) 55 rpm and (f) 65 rpm thin films. | ||
Fig. 2(a) and (b) (along with insets) show SEM images of NFs-ZnFe2O4 thin films obtained at 55 rpm rotation speed and NGs-ZnFe2O4 thin films obtained from 0 rpm rotation, respectively. The difference in morphology can be attributed to the trapping of MEA agent within the structures as a result of rotation of the substrate, which hindered attainment of equilibrium during deposition and growth of ZnFe2O4 thin films, unlike in case of conventional CBD with fixed substrate.23
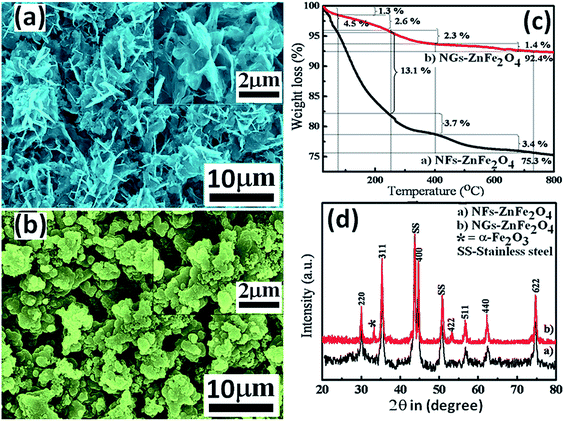 | ||
| Fig. 2 SEM images of (a) NFs-ZnFe2O4 and (b) NGs-ZnFe2O4. (c) TGA thermograms and (d) XRD patterns of NFs-ZnFe2O4 and NGs-ZnFe2O4 thin films. | ||
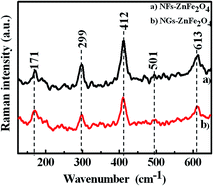
The observed significant variation in surface morphology and porous nature, probably as a result of trapping MEA and/or water molecules and observed thermal stability lead to curiosity, which is further justified from thermogravimetry analysis (TGA, 25–800 °C, 10 °C min−1 in air atmosphere) of sample scooped from NFs-ZnFe2O4 and NGs-ZnFe2O4 thin films (Fig. 2(c)). The TGA thermogram of NFs-ZnFe2O4 showed weigh loss in steps of 4.5 (H2O), 13.1, 3.7 and 3.4% as a function of temperature with total weight loss of 24.7% attributing to the loss of H2O24 and decomposition of MEA25 and oxidation of carbonaceous matter25 trapped into the pores of nano-flakes structures. On the other hand, the TGA thermogram of NGs-ZnFe2O4 showed weigh loss in steps of 1.3 (H2O), 2.6, 2.3 and 1.4% as a function of temperature with total weight loss of 7.6% attributing to the loss of H2O24 and decomposition of MEA25 and oxidation of carbonaceous matter25 trapped into the voids of nano-granular structures. The difference in weight loss indicates that the MEA and subsequent carbonaceous matter trapped in the pores of nano-flakes is more than in voids of nano-grains, justifying the difference in porosity and in agreement with observed morphology. Furthermore, the crystal structure and phase formation were characterized by X-ray diffraction (XRD). The XRD spectra recorded from both NFs-ZnFe2O4 and NGs-ZnFe2O4 thin films (Fig. 2(d)) confirmed the crystal structure to be spinel cubic, however, the spectrum obtained from NGs-ZnFe2O4 thin films showed presence of trace amount of α-Fe2O3. In Fig. 2(d), the peaks corresponding to bare stainless steel are marked as SS and the peaks at 2θ of 30.0° (220), 35.3° (311), 44.7° (400), 53.3° (422), 56.6° (511), 62.3° (440), and 74.7° (622) could be attributed to cubic spinel phase ZnFe2O4 (JCPDS 11-0325). The additional peak at 33.4° in NGs-ZnFe2O4 could be assigned to the α-Fe2O3 obtained from unreacted hydroxides of iron.3 The NFs-ZnFe2O4 and NGs-ZnFe2O4 thin films were characterized by Raman spectrophotometer and the resulting spectra is as shown in Fig. 3. Both the spectra revealed that NGs-ZnFe2O4 and NFs-ZnFe2O4 thin films have cubic structure that belongs to the space group Oh7(Fd3m)26,27 and have five first-order Raman active modes (A1g + Eg + 3T2g), and all these modes were observed at ambient conditions. In the cubic spinel including ferrites, the modes at above 600 cm−1 mostly correspond to the motion of oxygen in tetrahedral AO4 groups. So the mode at 613 cm−1 can be reasonably considered to have A1g symmetry. The other four first-order Raman modes at 299 (Eg), 171, 412, 501 cm−1 (T2g) corresponds to NGs-ZnFe2O4 and NFs-ZnFe2O4 thin films respectively. The all four first-order Raman modes (Eg + 3T2g) representing the characteristics of the octahedral sites (BO6). The obtained results were in good agreement with the literature reports.24,28
The difference in porosity was further confirmed by Brunauer–Emmett–Teller (BET) multipoint surface area and Barrett–Joyner–Halenda (BJH) pore size distribution analysis (Fig. 4(a) and (b)) of NFs-ZnFe2O4 (120 m2 g−1; the average pore distribution ∼1.7 nm) and NGs-ZnFe2O4 (67 m2 g−1, ∼2.5 nm).28
 | ||
| Fig. 4 N2 adsorption–desorption isotherms and pore size (nm) distribution (insets) of synthesized (a) NFs-ZnFe2O4 and (b) NGs-ZnFe2O4 thin films. | ||
Fig. 4(a) and (b) shows N2 adsorption–desorption isotherms of synthesized NFs-ZnFe2O4 and NGs-ZnFe2O4 thin films, respectively, and inset of corresponding pore size distributions (nm). For both cases, the isotherms shows discrete hysteresis loop starting from P/Po ∼ 0.45 indicating the presence of inter-connected as well as structurally open pores. The corresponding pore size distributions were calculated from the desorption part of the isotherms. Mesoporous nature with a combination of narrow and broad size distribution have been observed in both cases indicating coexistence of structural pores as well as inter-connected pores. BET multipoint surface area values are calculated to be 123 and 67 m2 g−1, respectively, for NFs-ZnFe2O4 and NGs-ZnFe2O4 nanostructures. A significant increase in the pore volume in NFs-ZnFe2O4 compared to the NFs-ZnFe2O4 can be related to presence of a large number of voids in its unique nano-flakes morphology.29,30
The elemental composition and chemicals state in NFs-ZnFe2O4 and NGs-ZnFe2O4 thin films was confirmed by XPS survey spectra (Fig. 5(a)) showing the presence of Zn, Fe and O with adventitious C confirming the formation of ZnFe2O4.31 Fig. 5(b) shows core level high resolution spectra of Zn2p wherein peaks corresponding to binding energy at 1021.58, 1021.38 and 1044.80, 1044.65 eV corresponding to Zn 2p3/2 and Zn 2p1/2, respectively, were observed for NFs-ZnFe2O4 and NGs-ZnFe2O4 samples. The oxidation state of the Zn was noted to be +2 in the prepared thin films. Fig. 5(c) shows high resolution spectra of Fe2p demonstrating the binding energy peaks at 711.46 and 710.90 for NFs-ZnFe2O4 and NGs-ZnFe2O4 thin films, respectively, due to Fe 2p3/2. The peak appearing at 725.15 and 724.97 eV could be attributed to Fe 2p1/2 for NFs-ZnFe2O4 and NGs-ZnFe2O4 thin films. The observed results were in good agreement with the earlier reports indicating the oxidation state of Fe to be +3. Furthermore, Fig. 5(d) shows high resolution O1s spectra with binding energy of 530.24 and 529.15 eV for NFs-ZnFe2O4 and NGs-ZnFe2O4, respectively, due to the lattice oxygen (O2−) in the prepared ZnFe2O4 thin film. The observed results show that there is small difference in binding energies of Fe2p, Zn2p and O1s between the NFs-ZnFe2O4 and NGs-ZnFe2O4 thin films. Binding energies of the NFs-ZnFe2O4 thin films are found to shift towards larger value as compared with NGs-ZnFe2O4 thin film sample because the arrangement of nano-flakes contain maximum number of reactive sites due to the mechanical force, while the nano-grains structures is expected to have minimum number of reactive sites.28,29,32
3.3 Supercapacitor study of NFs-ZnFe2O4 and NGs-ZnFe2O4 thin films
The supercapacitor application was studied by using electrochemistry, wherein three electrodes cell system was constructed. Fig. 6(a) shows cyclic voltammetry (CV) curves obtained from NFs-ZnFe2O4 and NGs-ZnFe2O4 thin films at 10 mV s−1 scan rate, wherein redox peaks were observed within the negative potential range from −1.4 to −0.3 V due to the Faradaic redox reactions related to M–O/M–O–OH, where M refers to Zn or Fe demonstrating pseudocapacitive behavior.33 The pseudocapacitive behaviour of ZnFe2O4 thin film is due to the intercalation and de-intercalation between Fe3+ and electrolytic K+ ion onto the porous electrode surface. Further, galvanostatic charge–discharge measurements from NFs-ZnFe2O4 and NGs-ZnFe2O4 thin films (Fig. 6(b)) were carried out within the potential window of −1.3 to −0.3 V vs. Ag/AgCl at 5 mA cm−2 current density, which showed maximum specific capacitance of 768 F g−1 and 534 F g−1, respectively, which is higher than earlier reports (Table 1). The capacitance value was derived from the discharge curves using eqn (3) and (4).
 | (3) |
 | (4) |
The electrochemical impedance spectra (EIS) with Nyquist plots are shown in Fig. 6(d). The impedance spectra obtained from NFs-ZnFe2O4 and NGs-ZnFe2O4 were observed to be similar showing a quasi-semicircle at a higher frequency region and a spike at lower frequency. The bulk solution resistance (Re), charge transfer resistance (Rct) and Warburg resistance (Zw) showed negligible difference in both samples at lower frequency. The Rct values were noted to be ∼3.0 Ω, indicating a very facile charge transfer in NFs-ZnFe2O4 and NGs-ZnFe2O4 thin films. The small Re value shown in Fig. 6(d) inset, further reveals good electrical conductivity of NFs-ZnFe2O4 and NGs-ZnFe2O4 thin films.34
The CV curves were also recorded at varying scan rate 10, 20, 40, 60, 80 and 100 mV s−1 from NFs-ZnFe2O4 (Fig. 7(a)) and NGs-ZnFe2O4 (Fig. 7(b)) thin films. The increase in scan rate increases the area under the curve which gives redox peaks at maximum current density. Hence the specific capacitance decreases. To further confirm the supercapacitive properties of NFs-ZnFe2O4 and NGs-ZnFe2O4 thin films as electrode material, the charge–discharge measurements were also carried out within the potential window −1.3 to −0.3 V vs. Ag/AgCl at different current densities 5 to 30 mA cm−2 for NFs-ZnFe2O4, while 5 to 20 mA cm−2 for NGs-ZnFe2O4 as shown in Fig. 7(c) and (d), respectively. The calculated specific capacitance for both films are 768, 391, 214, 183 F g−1 and 534, 245, 137, 95 F g−1 at current densities 5, 10, 20, 30 mA cm−2 and 5, 10, 15, 20 mA cm−2, respectively. It can be observed that the calculated specific capacitances of both the electrodes of ZnFe2O4 thin films decreased along with the increase in current density (Fig. 7(e)). At high current density, the low diffusion of electrolytic cation (K+) reduces the specific capacitance. The ionic motion in the electrolyte is always limited by diffusion because of the time constraint during the high-rate of charge–discharge process, and only the outer active surface is utilized for charge storage.
Finally, the energy density (W h kg−1) and power density (kW kg−1) were calculated using following eqn (5) and (6).
 | (5) |
 | (6) |
4 Conclusions
In summary, the present work demonstrates the development of a simple, economic, easy to handle, scalable and reproducible soft mechanochemical (R-CBD) deposition technique for the deposition of binder free porous spinel NFs-ZnFe2O4 thin films showing excellent performance as promising electrode materials in supercapacitor. The NFs-ZnFe2O4 thin films (768 F g−1) showed better pseudocapacitive behavior than NGs-ZnFe2O4 thin films (534 F g−1) in 3 M KOH aqueous electrolyte, within the potential window (−1.3 to −0.3 V) at current density of 5 mA cm−2 and are higher than those reported earlier for MFe2O4. The capacitance retention was noted to be 88% even after 5000 cycles demonstrating good cycle stability. Therefore, employing R-CBD as soft chemical synthetic route to assemble porous electrodes demonstrating high specific capacitance may open new avenue to engineer other promising materials for improved electrochemical properties with great promise in energy storage device applications.Notes and references
- T. Brezesinski, J. Wang, S. H. Tolbert and B. Dunn, Nat. Mater., 2010, 9, 146–151 CrossRef CAS PubMed.
- Z. S. Wu, Y. Sun, Y. Z. Tan, S. Yang, X. Feng and K. Müllen, J. Am. Chem. Soc., 2012, 134, 19532–19535 CrossRef CAS PubMed.
- X. Yao, C. Zhao, J. Kong, H. Wu, D. Zhou and X. Lu, Chem. Commun., 2014, 50, 14597–14600 RSC.
- Z. C. Yang, C. H. Tang, Y. Zhang, H. Gong, X. Li and J. Wang, Sci. Rep., 2013, 3, 2595 Search PubMed.
- H. Wang, J. Guo, C. Qing, D. Sun, B. Wang and Y. Tang, Chem. Commun., 2014, 50, 8697–8700 RSC.
- D. Zhao, Y. Xiao, X. Wang, Q. Gao and M. Cao, Nano Energy, 2014, 7, 124–133 CrossRef CAS PubMed.
- Q. Zhou, J. Xing, Y. Gao, X. Lv, Y. He, Z. Guo and Y. Li, ACS Appl. Mater. Interfaces, 2014, 6, 11394–11402 CAS.
- S. Wang, J. Pu, Y. Tong, Y. Cheng, Y. Gao and Z. Wang, J. Mater. Chem. A, 2014, 2, 5434–5440 CAS.
- V. S. Kumbhar, A. D. Jagadale, N. M. Shinde and C. D. Lokhande, Appl. Surf. Sci., 2012, 259, 39–43 CrossRef CAS PubMed.
- J. L. Gunjakar, A. M. More, K. V. Gurav and C. D. Lokhande, Appl. Surf. Sci., 2008, 254, 5844–5848 CrossRef CAS PubMed.
- J. Chen, K. Huang and S. Liu, Electrochim. Acta, 2009, 55, 1–5 CrossRef CAS PubMed.
- S. L. Kuo and N. L. Wu, Electrochem. Solid-State Lett., 2005, 8, A495–A499 CrossRef CAS PubMed.
- M. Zhu, D. Meng, C. Wang and G. Diao, ACS Appl. Mater. Interfaces, 2013, 5, 6030–6037 CAS.
- Y. Zhang and Z. Guo, Chem. Commun., 2014, 50, 3443–3446 RSC.
- M. C. Liu, L. B. Kong, C. Lu, X. M. Li, Y. C. Luo and L. Kang, ACS Appl. Mater. Interfaces, 2012, 4, 4631–4636 CAS.
- S. Vijayakumar, S. Nagamuthu and G. Muralidharan, ACS Sustainable Chem. Eng., 2013, 1, 1110–1118 CrossRef CAS.
- Z. Y. Yu, L. F. Chen and S. H. Yu, J. Mater. Chem. A, 2014, 2, 10889–10894 CAS.
- B. Guan, D. Guo, L. Hu, G. Zhang, T. Fu, W. Ren, J. Li and Q. Li, J. Mater. Chem. A, 2014, 2, 16116–16123 CAS.
- G. Zhang and X. W. Lou, Sci. Rep., 2013, 3, 1470 Search PubMed.
- D. K. Pawar, S. M. Pawar, P. S. Patil and S. S. Kolekar, J. Alloys Compd., 2011, 509, 3587–3591 CrossRef CAS PubMed.
- D. K. Pawar, J. S. Shaikh, B. S. Pawar, S. M. Pawar, P. S. Patil and S. S. Kolekar, J. Porous Mater., 2012, 19, 649–655 CrossRef CAS PubMed.
- L. Wang, H. Ji, S. Wang, L. Kong, X. Jiang and G. Yang, Nanoscale, 2013, 5, 3793–3799 RSC.
- Z. Yu, Z. Cheng, S. R. Majid, Z. Tai, X. Wang and S. Dou, Chem. Commun., 2015, 51, 1689–1692 RSC.
- M. Wang, Z. Ai and L. Zhang, J. Phys. Chem. C, 2008, 112, 13163–13170 CAS.
- G. Nam, B. Kim, Y. Park, S. Park, J. Moon, D. Y. Kim, S. O. Kim and J. Y. Leem, J. Mater. Chem. C, 2014, 2, 9918–9923 RSC.
- K. Mukherjee and S. B. Majumder, Sens. Actuators, B, 2012, 162, 229–236 CrossRef CAS PubMed.
- O. Akhavan, A. Meidanchi, E. Ghaderi and S. Khoei, J. Mater. Chem. B, 2014, 2, 3306–3314 RSC.
- H. Lv, L. Ma, P. Zeng, D. Ke and T. Peng, J. Mater. Chem., 2010, 20, 3665–3672 RSC.
- A. Shanmugavani and R. K. Selvan, RSC Adv., 2014, 4, 27022–27029 RSC.
- R. Indhrajothi, I. Prakash, M. Venkateswarlu and N. Satyanarayana, RSC Adv., 2014, 4, 44089–44099 RSC.
- X. Hou, X. Wang, L. Yao, S. Hu, Y. Wu and X. Liu, New J. Chem., 2015, 39, 1943–1952 RSC.
- Y. Zhang, Q. Shi, J. Schliesser, B. F. Woodfield and Z. Nan, Inorg. Chem., 2014, 53, 10463–10470 CrossRef CAS PubMed.
- J. W. Lee, A. S. Hall, J.-D. Kim and T. E. Mallouk, Chem. Mater., 2012, 24, 1158–1164 CrossRef CAS.
- Z. Wang, X. Zhang, Y. Li, Z. Liu and Z. Hao, J. Mater. Chem. A, 2013, 1, 6393–6399 CAS.
- Q. Tang, W. Wang and G. Wang, J. Mater. Chem. A, 2015, 3, 6662–6670 CAS.
- J. Hao, W. Yang, Z. Zhang, B. Lu, B. Zhang and J. Tang, Electrochim. Acta, 2015, 152, 13–18 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |