Substrate selective synthesis of pyrazolo[1,5-a]pyridines through [3 + 2] cycloaddition of N-aminopyridines and β-nitro styrenes†
Chitrakar Ravi,
Darapaneni Chandra Mohan,
N. Naresh Kumar Reddy and
Subbarayappa Adimurthy*
Academy of Scientific & Innovative Research, CSIR – Central Salt & Marine Chemicals Research Institute, G. B. Marg, Bhavnagar-364 002, Gujarat, India. E-mail: adimurthy@csmcri.org
First published on 23rd April 2015
Abstract
Synthesis of (hetero)aryl pyrazolo[1,5-a]pyridines through [3 + 2] cycloaddition of N-aminopyridine with β-nitrostyrenes followed by in situ denitration and oxidation under metal-free, mild conditions are described.
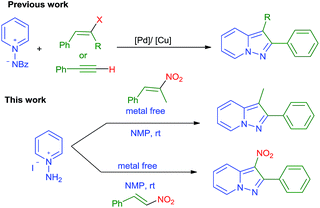
Pyrazolopyridines (PP) are a class of fused heteroaromatic bicyclic compounds and have attracted considerable attention as a privileged structural unit in chemistry and biology.1 Their derivatives exhibit remarkable bioactivities including 5HT3-antagonists,2 p38 kinase inhibitors,3 dopamine D3/D4 agonists and antagonists 2,4-anti-herpetic agents,4 and potent treatments for cardiac arrhythmias (Fig. 1).
Because of the importance of these molecules, some graceful methods have been developed, which include the regioselective [3 + 2] cycloaddition of N-aminopyridines with alkynes bearing electron-withdrawing groups,5,6 domino direct alkynylation followed by cyclization of N-iminopyridinium ylides,6 direct oxidative annulation of N-iminopyridinium ylides with terminal alkynes,7 nitrene insertion,8 rearrangements of pyridine derivatives bearing aziridine groups and other methods.9
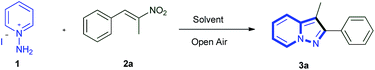
Recently, nitro olefins have been used as coupling partners for the synthesis of heterocyclic compounds as they are excellent substrates for 1,4-addition reactions due to the strong electron-withdrawing ability of the nitro group and low tendency for 1,2-addition.10 In addition nitro alkenes are bielectrophilic in nature and they are potentially useful for multiple nucleophilic additions to form heterocycles in a cascade fashion.11 In continuation of our interest on the synthesis of fused heterocyclic compounds,12 here we report metal-free and base-free synthesis of pyrazolo[1,5-a]pyridines through [3 + 2] cycloaddition of N-aminopyridine with β-nitrostyrenes followed by in situ denitration and oxidation under mild conditions (Scheme 1). To the best of our knowledge this is first report on metal and base-free conditions.
Initially, we investigated the reaction of N-aminopyridine with β-nitrostyrenes as a potential route to pyrazolo[1,5-a]pyridines. Our initial experimental efforts focused on the development of conditions for the annulation reaction of N-aminopyridine and β-nitrostyrene as these starting substrates are commercially available. Initially, we performed the reaction of 1-aminopyridinium iodide 1 (1.0 equiv.) and (E)-(2-nitroprop-1-en-1-yl) benzene 2a (1.0 equiv.), CuI (0.1 equiv.) as a catalyst at room temperature in DMF, but no product formation was observed (Table 1, entry 1). When the same reaction was performed at 80 °C, 3a was obtained in 60% yield (Table 1, entry 2). The yield decreased with other copper halides (Br and Cl) (Table 1, entries 3 and 4). To our delight when the reaction was conducted without a copper source a 62% yield of 3a was still obtained (Table 1, entry 5). Inspired by the yield obtained under metal-free conditions, the reaction was performed in DMSO and NMP as solvent, and 66% and 68% yield of 3a was obtained respectively (Table 1, entries 6 and 7). Then the reaction in NMP was performed at room temperature, and 73% of desired product 3a was obtained (Table 1, entry 8). Further, the yield of 3a was raised up to 78% and 83% by increasing the amount of 1 to 1.2 and 1.5 equiv. (w.r.t. 2a) respectively (Table 1, entries 9 and 10). Furthermore, the yield did not improve either by increasing the NMP or decreasing the reaction time and with other solvents (H2O, MeOH, TEA, DMA, toluene, and chlorobenzene) screened out (Table 1, entries 11–21). A low yield was observed under oxygen and argon atmospheres (Table 1, entry 22 and 23). The product 3a was further confirmed by XRD analysis (Fig. 2). Therefore, the optimum conditions set for the present protocol are as follows: NMP as solvent, open air, at room temperature, 24 h reaction time (Table 1, entry 10).
| Entry | Catalyst | Solvent (1 mL) | Temp (°C) | Time (h) | Yield (%) |
|---|---|---|---|---|---|
a Conditions: 0.3 mmol 1a, 0.2 mmol 2a, NMP (1 mL), 24 hours, room temperature, open air isolated yields.b 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 of 1a and 2a.c 5 mL(NMP).d O2-balloon.e Argon atmosphere. 1 of 1a and 2a.c 5 mL(NMP).d O2-balloon.e Argon atmosphere. |
|||||
| 1b | CuI | DMF | Rt | 24 | Nr |
| 2b | CuI | DMF | 80 | 24 | 60 |
| 3b | CuBr | DMF | 80 | 24 | 55 |
| 4b | CuCl | DMF | 80 | 24 | 40 |
| 5b | — | DMF | 80 | 24 | 62 |
| 6b | — | DMSO | 80 | 24 | 66 |
| 7b | — | NMP | 80 | 24 | 68 |
| 8b | — | NMP | Rt | 24 | 73 |
| 9b | — | NMP | Rt | 24 | 78 |
| 10 | — | NMP | Rt | 24 | 83 |
| 11c | — | NMP | Rt | 24 | 64 |
| 12 | — | NMP | Rt | 1 | 27 |
| 13 | — | NMP | Rt | 6 | 39 |
| 14 | — | NMP | Rt | 12 | 42 |
| 15 | — | NMP | Rt | 36 | 83 |
| 16 | — | H2O | Rt | 24 | — |
| 17 | — | MeOH | Rt | 24 | — |
| 18 | — | TEA | Rt | 24 | — |
| 19 | — | DMA | Rt | 24 | — |
| 20 | — | Toluene | Rt | 24 | — |
| 21 | — | Chloro benzene | Rt | 24 | — |
| 22d | — | NMP | Rt | 24 | 53 |
| 23e | — | NMP | Rt | 24 | 49 |
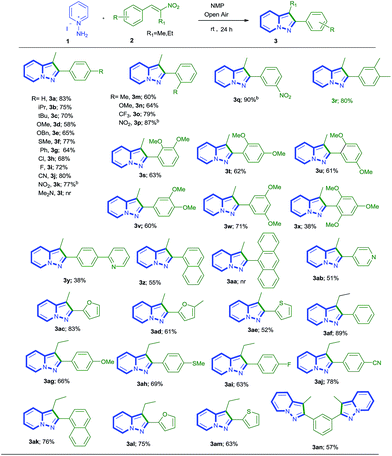
Under this set of optimized conditions (Table 1, entry 10), the scope of the transition metal-free [3 + 2] cycloaddition reaction of 1 with nitrostyrenes 2 for the synthesis of pyrazolo[1,5-a]pyridines 3 was then investigated (Table 2). The reaction was found to be very facile with both electron-rich and deficient nitrostyrenes. The presence of a variety of electron-donating/withdrawing groups in the nitrostyrene moieties at the para-position could be tolerated and the reactions afforded the desired PPs 3a–3k in 58–83% yield. However, the presence of a strong electron releasing group like N,N-dimethyl at the para-position did not afford the product (3l). Similarly the presence of these groups at the ortho/meta position of styrene derivatives also afforded the desired products 3n–3x in moderate to good yields (60–90%). As can be seen from the yields of products 3l (with strong electron releasing group; NMe2) and 3q (with strong electron withdrawing group; NO2), both are in controversial to each other. The tri-methoxy and 2-pyridyl substituted styrenes gave the desired products 3x and 3y respectively in 38% yield each. Then the methodology was extended to bicyclic (Z)-1-(2-nitroprop-1-en-1-yl)naphthalene and tricyclic (E)-9-(2-nitroprop-1-en-1-yl)anthracene derivatives, in the former case 55% yield of desired product 3z was obtained and in the latter one (3aa) there was no reaction. Interestingly, important heteroatom containing nitro olefins like pyridine, furan, thiofuran derivatives reacted smoothly and gave the pyrazolo[1,5-a]pyridine derivatives 3ab–3ae in good to moderate yields (51–83%). Then we focused on the reaction of 1 with ethyl containing nitro olefin [(E)-(2-nitrobut-1-en-1-yl)benzene] instead of methyl nitro olefins, both electron releasing/withdrawing substituents irrespective of position (o/m/p) and also heteroatom olefins, which reacted smoothly and afford the desired products 3af–3am with yields ranging from 51% to 78%.
In order to verify the influence of substituents on β-nitrostyrene, we performed the reaction of (E)-(2-nitrovinyl)benzene 4a and 1; surprisingly 84% 3-nitro-2-phenylpyrazolo[1,5-a]pyridine 5a was isolated instead of 2-phenylpyrazolo[1,5-a]pyridine 6 (Scheme 2). Based upon this observation, we then searched the literature reports for such molecules, but no methods exist to get 3-nitro-2-phenylpyrazolo[1,5-a]pyridine 5a. Then under the optimized conditions we synthesized substrate selective 3-nitro-2-phenylpyrazolo[1,5-a]pyridineidines (Table 3). The reaction was found to be very facile with both electron rich and electron-deficient β-nitrostyrenes. The reaction of N-aminopyridine (1) with simple electron rich β-nitro styrenes, for example tBu, dimethyl, OMe, and SMe β-nitro styrenes reacted smoothly and gave the desired products 5a–5e in moderate to good yields. The reaction of electron-withdrawing substituents of 4, led to the corresponding products 5f and 5g in 77% and 52% yields respectively. This system is also applicable for bicyclic and hetero-β-nitrostyrenes and provided the desired products 5h and 5j in 50% and 69% yields.
| a Reaction conditions 0.3 mmol 1a, 0.2 mmol 2a, NMP (1 mL), 24 hours, room temperature, open air at 60 °C, isolated yields. |
|---|
 |
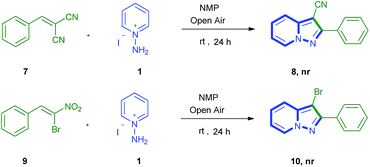
For the readership understanding, we performed some additional experiments (Scheme 3). The reaction of 2-benzylidenemalononitrile (7) was subjected to the optimized conditions with 1, but no desired product was observed (7 was decomposed). When β-bromo-β-nitrostyrene 9 instead of 2a was reacted with 1, no desired product 10 formation was observed (9 was decomposed).
Based upon the above observation, we proposed a plausible reaction mechanism in Scheme 4. Initially, N-aminopyridine 1 reacts with β-nitrostyrene 2a and 4a individually through a [3 + 2] cycloaddition pathway and generates the corresponding intermediates A and B. Denitration of A, and oxidation of B, generates intermediates C and D respectively. Finally, aerobic oxidation of these intermediates (C and D) yield the desired products 3a and 5a respectively.
In conclusion, we have developed a new protocol for the substrate selective synthesis of pyrazolo[1,5-a]pyridines through [3 + 2] cycloaddition reactions of N-aminopyridine with β-nitrostyrenes under metal-free conditions. Methyl substituted β-nitrostyrenes gave methyl substituted pyrazolo[1,5-a]pyridines through denitration and unsubstituted β-nitro styrenes gave nitro substituted pyrazolo[1,5-a]pyridines through double oxidation. The presence of strong electron withdrawing β-nitrostyrenes gave excellent yields under mild reaction temperature (65 °C). The method is also applicable for other β-nitro vinyl (hetero)arenes like (Z)-4-(2-nitroprop-1-en-1-yl)pyridines, (Z)-2-(2-nitroprop-1-en-1-yl)thiophenes and (Z)-2-methyl-5-(2-nitroprop-1-en-1-yl)furans.
Acknowledgements
CSIR-CSMCRI Communication no. 003/2015. D.C.M & C.R. are thankful to AcSIR for their Ph.D. enrolment and the “Analytical Discipline and Centralized Instrumental Facilities” for providing instrumentation facilities. D.C.M. is also thankful to UGC, New Delhi for his fellowship. We thank DST, Government of India (SR/S1/OC-13/2011), for financial support. We also thank CSIR-CSMCRI (OLP-0076) for partial assistance.Notes and references
- (a) T. Eicher and S. Hauptmann, The Chemistry of Heterocycles: Structures, Reactions, Synthesis, and Applications, Wiley-VCH, Weinheim, 2nd edn, 2003 Search PubMed; (b) S. Lober, H. Hubner and P. Gmeiner, Bioorg. Med. Chem. Lett., 2002, 12, 2377 CrossRef CAS; (c) J. B. Hansen, J. Weis, P. D. Suzdak and K. Eskesen, Bioorg. Med. Chem. Lett., 1994, 4, 695 CrossRef; (d) L. Bettinetti, K. Schlotter, H. Hubner and P. Gmeiner, J. Med. Chem., 2002, 45, 4594 CrossRef CAS PubMed; (e) Y. Takahashi, S. Hibi, Y. Hoshino, K. Kikuchi, K. Shin, K. Murata-Tai, M. Fujisawa, M. Ino, H. Shibata and M. Yonaga, J. Med. Chem., 2012, 55, 5255 CrossRef CAS PubMed; (f) J. D. Kendall, A. J. Marshall, A. C. Giddens, K. Y. Tsang, M. Boyd, R. Frederick, C. L. Lill, W.-J. Lee, S. Kolekar, M. Chao, A. Malik, S. Yu, C. Chaussade, C. M. Buchanan, G. W. Rewcastle, B. C. Baguley, J. U. Flanagan, W. A. Dennya and P. R. Shepherd, MedChemComm, 2014, 5, 41 RSC.
- B. J. Hansen, J. Weis, P. D. Suzdak and K. Eskesen, Bioorg. Med. Chem. Lett., 1994, 4, 695 CrossRef.
- K. L. Stevens, D. K. Jung, M. J. Alberti, J. G. Badiang, G. E. Peckham, J. M. Veal, M. Cheung, P. A. Harris, S. D. Chamberlain and M. R. Peel, Org. Lett., 2005, 7, 4753 CrossRef CAS PubMed.
- (a) S. Löber, H. Hübner, W. Utz and P. Gmeiner, J. Med. Chem., 2001, 44, 2691 CrossRef PubMed; (b) L. Bettinetti, K. Schlotter, H. Hübner and P. Gmeiner, J. Med. Chem., 2002, 45, 4594 CrossRef CAS PubMed; (c) S. Löber, T. Aboul-Fadl, H. Hübner and P. Gmeiner, Bioorg. Med. Chem. Lett., 2002, 12, 633 CrossRef; (d) S. Löber, B. Ortner, L. Bettinetti, H. Hübner and P. Gmeiner, Tetrahedron: Asymmetry, 2002, 13, 2303 CrossRef; (e) F. Boeckler, H. Russig, W. N. Zhang, S. Löber, J. Schetz, H. Hübner, B. Ferger, P. Gmeiner and J. Feldon, Psychopharmacology, 2004, 175, 7 CrossRef CAS PubMed; (f) L. Bettinetti, H. Hübner and P. Gmeiner, Arch. Pharm., 2005, 338, 276 CrossRef CAS PubMed; (g) O. Prante, C. Hocke, S. Löber, H. Hübner, P. Gmeiner and T. Kuwert, Nuklearmedizin, 2006, 45, 41 CAS.
- (a) S. Lober, H. Hubner and P. Gmeiner, Bioorg. Med. Chem. Lett., 1999, 9, 97 CrossRef CAS; (b) K. L. Stevens, D. K. Jung, M. J. Alberti, J. G. Badiang, G. E. Peckham, J. M. Veal, M. Cheung, P. A. Harris, S. D. Cham-berlain and M. R. Peel, Org. Lett., 2005, 7, 4753 CrossRef CAS PubMed; (c) X. Yu, S. Ye and J. Wu, Adv. Synth. Catal., 2010, 352, 2050 CrossRef CAS PubMed; (d) Z. Chen, M. Su, X. Yu and J. Wu, Org. Biomol. Chem., 2009, 7, 4641 RSC; (e) K. A. Johnston, R. W. Allcock, Z. Jiang, I. D. Collier, H. Blakli, G. M. Rosair, P. D. Bailey, K. M. Morgan, Y. Kohno and D. R. Adams, Org. Biomol. Chem., 2008, 6, 175 RSC; (f) K. Harju, I. Kylanlahti, T. Paananen, M. Polamo, J. Nielsen and J. Yli-Kauhaluoma, J. Comb. Chem., 2006, 8, 344 CrossRef CAS PubMed; (g) S. Patnaik, H. C. Dietz, W. Zheng, C. Austin and J. J. Marugan, J. Org. Chem., 2009, 74, 8870 CrossRef CAS PubMed; (h) Y. Miki, J. Tasaka, K. Uemura, K. Miyazeki and J. Yamada, Heterocycles, 1996, 43, 2249 CrossRef CAS; (i) Z. Chen and J. Wu, Org. Lett., 2010, 12, 4856 CrossRef CAS PubMed; (j) Y. Hoashi, T. Takai, E. Kotani and T. Koike, Tetrahedron Lett., 2013, 54, 2199 CrossRef CAS PubMed.
- (a) J. Zhao, C. Wu, P. Li, W. Ai, H. Chen, C. Wang, R. C. Larock and F. Shi, J. Org. Chem., 2011, 76, 6837 CrossRef CAS PubMed; (b) Z. Chen and J. Wu, Org. Lett., 2010, 12, 4856 CrossRef CAS PubMed; (c) J. Zhao, P. Li, C. Wu, H. Chen, W. Ai, R. Sun, R. Ren, R. C. Larock and F. Shi, Org. Biomol. Chem., 2012, 10, 1922 RSC.
- (a) J. J. Mousseau, A. Fortier and A. Charette, Org. Lett., 2010, 12, 516 CrossRef CAS PubMed; (b) J. J. Mousseau, J. A. Bull, C. L. Ladd, A. Fortier, S. D. Roman and A. B. Charette, J. Org. Chem., 2011, 76, 8243 CrossRef CAS PubMed.
- (a) S. Ding, Y. Yan and N. Jiao, Chem. Commun., 2013, 49, 4250 RSC; (b) L. Ling, J. Chen, J. Song, Y. Zhang, X. Li, L. Song, F. Shi, Y. Li and C. Wu, Org. Biomol. Chem., 2013, 11, 3894 RSC; (c) Z. Chen, M. Su, X. Yua and J. Wu, Org. Biomol. Chem., 2009, 7, 4641 RSC; (d) P. Huang, Q. Yang, Z. Chen, Q. Ding, J. Xu and Y. Peng, J. Org. Chem., 2012, 77, 8092 CrossRef CAS PubMed.
- D. Csanyi, G. Hajos, Z. Riedl, G. Timari, Z. N. Bajor, F. Co-chard, J. Sapi and J.-Y. Laronze, Bioorg. Med. Chem. Lett., 2000, 10, 1767 CrossRef CAS.
- B. A. Johns, K. S. Gudmundsson, E. M. Turner, S. H. Allen, D. K. Jung, C. J. Sexton, F. L. Boyd and M. R. Peel, Tetrahedron, 2003, 59, 9001 CrossRef CAS PubMed.
- (a) G. Rosini and R. Ballini, Synthesis, 1988, 833 CrossRef CAS; (b) N. Ono, in The Nitro Group in Organic Synthesis, Wiley-VCH, New York, 2001 CrossRef PubMed; (c) R. Ballini, G. Bosica, D. Fiorini, A. Palmieri and M. Petrini, Chem. Rev., 2005, 105, 933 CrossRef CAS PubMed; (d) S. Santra, A. K. Bagdi, A. Majee and A. Hajra, Adv. Synth. Catal., 2013, 355, 1065 CrossRef CAS PubMed.
- (a) K. C. Chunavala, G. Joshi, E. Suresh and S. Adimurthy, Synthesis, 2011, 635 CAS; (b) D. C. Mohan, S. N. Rao and S. Adimurthy, J. Org. Chem., 2013, 78, 1266 CrossRef PubMed; (c) D. C. Mohan, R. R. Donthiri, S. N. Rao and S. Adimurthy, Adv. Synth. Catal., 2013, 355, 2217 CrossRef PubMed; (d) D. C. Mohan, N. B. Sarang and S. Adimurthy, Tetrahedron Lett., 2013, 54, 6077 CrossRef PubMed; (e) D. C. Mohan, S. N. Rao, C. Ravi and S. Adimurthy, Asian J. Org. Chem., 2014, 3, 609 CrossRef CAS PubMed; (f) D. R. Reddy, P. Venkatanarayana, N. N. K. Reddy, D. Bairagi and S. Adimurthy, J. Org. Chem., 2014, 79, 11277 CrossRef PubMed; (g) D. C. Mohan, C. Ravi, S. N. Rao and S. Adimurthy, Org. Biomol. Chem., 2015, 13, 3556 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: 1H and 13C NMR spectra for all compounds. Crystallographic data for compound 3a. CCDC 1015855. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra06707c |
| This journal is © The Royal Society of Chemistry 2015 |