Novel aligned sodium vanadate nanowire arrays for high-performance lithium-ion battery electrodes†
Abstract
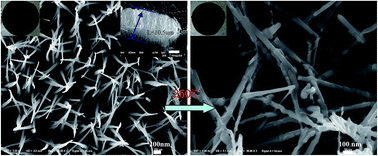
Sodium vanadate (Na5V12O32 or Na1.25V3O8) nanowire arrays were successfully prepared using a facile hydrothermal method with subsequent calcination. The length of the Na5V12O32 nanowire arrays on titanium foil were about 10.5 μm. The unique architecture renders a high-rate transportation of lithium ions that is attributed to their nanosized structure, active materials connected to the current collector and the high specific surface area. The Na5V12O32 nanowire arrays on titanium foil annealed at 250 °C as electrodes for lithium-ion batteries exhibit a significant capacity stability with a capacity from 339.3 to 289.7 mA h g−1 in 50 cycles at 50 mA g−1. The superior electrochemical performance demonstrated that the Na5V12O32 nanowire arrays are promising electrodes for secondary organic lithium-ion batteries.

 Please wait while we load your content...
Please wait while we load your content...