A sensitive cataluminescence-based sensor using a SrCO3/graphene composite for n-propanol
Qianchun Zhang*,
Feifei Meng,
Lin Zha,
Xingyi Wang and
Guoyi Zhang
School of Biology and Chemistry, Xingyi Normal University for Nationalities, Xingyi, 562400, People's Republic of China. E-mail: qianchunzhang@qq.com; Fax: +86-859-3527567; Tel: +86-589-3296359
First published on 15th June 2015
Abstract
In this paper, we developed a cataluminescence-based sensor using SrCO3/graphene for sensitive and selective detection of n-propanol. The composite was characterized by X-ray diffraction, transmission electron microscopy, Fourier transform infrared spectroscopy, and gas adsorption. The sensor was coupled with a miniature vaporizing device to detect n-propanol in liquid samples. The experimental results revealed that the SrCO3/graphene sensor exhibited a sensitivity for n-propanol 5.8 times higher than that of pure SrCO3, indicating that the sensitivity of the SrCO3/graphene sensor was increased by adding graphene to SrCO3. The linear range of the sensor was 0.2 to 32 mg L−1 (r = 0.9987) with a limit of detection of 0.08 mg L−1. The sensor showed a rapid response of 2 s and a recovery time of 20 s, respectively. The sensor was used to analyze samples spiked with known concentrations of n-propanol. The concentrations of n-propanol in all samples were well quantified with satisfactory recoveries, indicating that the SrCO3/graphene sensor is a promising candidate for fast, sensitive, selective detection of n-propanol. We also discuss the possible mechanism based on the reaction products.
1. Introduction
Graphene, which is a two-dimensional monolayer of fused sp2 carbon bonds in a honeycomb-like network, has attracted a great deal of scientific interest for chemical gas sensing because of its outstanding mechanical, electrical, thermal, and optical properties, and large specific surface area.1–4 Graphene is an ideal choice for decorating with other materials, including metal oxides, metal sulfides and metal nanoparticles to improve its properties.5–7 It has been reported that mixing graphene into other materials can enhance the sensitivity and improve the selectivity of the composite, and also reduce the operating temperature.8–10 For example, Singh et al. reported that the sensors based on ZnO-decorated graphene can sensitively detect industrial gases, such as CO, NH3, and NO, at room temperature.11 Liu et al. reported that mixing graphene into ZnFe2O4 can reduce the operating temperature of the sensors for acetone.12Cataluminescence (CTL) is emitted during the catalytic oxidation of an analyte molecule on the surface of a solid catalyst.13–15 CTL has attracted a great deal of research interest in the development of chemical sensors for quantifying and distinguishing analytes.16–19 The CTL-based gas sensor possesses many outstanding advantages, such as good selectivity, high sensitivity, rapid response speed, good reproducibility, and simple instrumentation.20–22 The sensing element of the CTL sensor is a solid catalyst that is not consumed during sensing, and thus this kind of chemical sensor has long-term stability.23,24 To date, many different kinds of materials have been used to develop CTL-based sensors. Examples include an isobutanol sensor based on a Y-doped metal–organic framework-5,25 an n-hexane sensor based on zeolite,26 a nanosized ZrO2-based sensor for propionaldehyde,27 and a nano-3TiO2–2BiVO4-based sensor for simultaneously detecting benzene and formaldehyde.28 Lv and co-workers reported a facile hydrothermal assisted in situ synthesis route for preparing graphene sheets decorated with SnO2. The SnO2/graphene composite was a highly efficient material for a CTL propanal sensor.29 Lv and co-workers also reported a facile catalyst-free atmospheric pressure chemical vapor deposition method for growing hierarchical SnO2 architectures on graphene.7 The materials prepared by this method showed an enhanced CTL response to methanol and morphology-dependent CTL performance.
n-Propanol is a colorless, flammable, fragrant liquid with moderate toxicity that is ubiquitous in nature. It is an important solvent, principally used in printing, cosmetics, leather products, and pesticides.30 In addition, n-propanol is the main higher alcohol in alcoholic beverages because it is widely used as a flavor volatile in food and beverage manufacturing.31 However, exposure to n-propanol can irritate the skin, causing a rash or burning feeling on contact, and higher exposure can cause headache, dizziness, confusion, nausea, vomiting, and even liver damage.32 Moreover, n-propanol is a flammable liquid and a dangerous fire hazard that can cause explosions when exposed to an open flame. Recently, increasing concerns have focused on levels of higher alcohols, including n-propanol, in surrogate alcohol (i.e., illicit or home-brewed alcoholic beverages) that might result in an increased incidence of liver disease in regions where there is a high consumption of such beverages.31 Therefore, sensors for n-propanol have wide applications in environmental monitoring and foodstuff control.
In this work, SrCO3 doped with 12 wt% graphene exhibited a CTL response to n-propanol 5.8 times greater than that of pure SrCO3. A CTL-based sensor using SrCO3/graphene was designed for sensitive and selective detection of n-propanol. Although graphene has been widely used in the design of electrochemical sensors, there are few studies of its use in CTL sensors. Moreover, previous CTL-based sensors were generally used for gaseous sample analysis. To expand the range of suitable samples, a miniature vaporizing device was coupled with the sensor cell for analyzing n-propanol in liquid samples; therefore, we focused on liquid sample sensing. The performance of the sensor was evaluated systematically in terms of response and recovery times, selectivity for n-propanol, and stability. The factors affecting the sensor for determining n-propanol were optimized. The potential application of the sensor was demonstrated by determining n-propanol in spiked samples. The results showed that the sensor provides a simple, rapid, sensitive method for determining n-propanol.
2. Experimental
2.1 Chemicals and materials
All chemicals used in our experiments were of analytical grade. Methanol, ethanol, ethyl acetate, n-hexane, formaldehyde, carbon tetrachloride, chloroform, propionaldehyde, acetaldehyde, 2-propanol, and acetone were purchased from Damao Chemical Reagent Company (Tianjin, China). n-Heptane, n-octane, and isooctane were supplied by Fuyu Fine Chemical Co., Ltd. (Tianjin, China). Hydrogen sulfide (500 ppm) was purchased from Zhandong Gas Co., Ltd. (Chongqing, China). Graphite powder (particle size 300–500 nm) was purchased from Alfa Aesar (Ward Hill, MA, USA). SrCO3 was supplied by XinxinYuan Chemical Co., Ltd. (Sichuan, China). Other chemicals were obtained from Aladdin Chemical Co., Ltd. (Shanghai, China).2.2 Material preparation and characterization
Graphene was synthesized by hydrazine reduction of graphene oxide (GO). The GO was prepared by oxidizing graphite powder with H2SO4/KMnO4 according to the modified Hummers' method,33,34 which resulted in water-soluble GO. Then, GO (0, 3, 12, 24, 30, 36, 45, and 54 mg) was dispersed in distilled water by ultrasonication to form colloidal suspensions. Subsequently, SrCO3 powder (0.3 g) was added to prepare composites containing different graphene contents. After 30 min ultrasonication, hydrazine monohydrate was added to the solution, which was then ultrasonicated for 30 min, and then refluxed at 90 °C for 6 h to ensure the complete reduction of GO. The solid products were filtered off with a 0.45 mm filter and washed with distilled water until they were neutral. The precipitate was dried at 80 °C for 24 h under vacuum.The morphologies of the materials were characterized with a transmission electron microscope (TEM; G2 Spirit, Tecnai) at an accelerating voltage of 120 kV. X-ray power diffraction (XRD) experiments were carried out on a Thermo-VG Scientific ESCALAB 250 diffractometer. Fourier transform infrared (FT-IR) spectra were measured by Nicolet Avatar 330. Surface area measurements were performed on an ASAP-2020M gas adsorption instrument (Micromeritics, Atlanta, USA) at 77 K.
2.3 CTL sensor fabrication and measurement
Fig. 1 shows a schematic diagram of the sensor. The SrCO3/graphene composite (0.1 g) was deposited onto a cylindrical ceramic heater (length = 10 cm, diameter = 0.6 cm) to form a catalyst layer. The ceramic heater was linked to a voltage controller (Power I, TG1783SL3A, Tonggao Electronic Co., Ltd., Ningbo, China). The temperature of the ceramic heater was controlled by adjusting the output voltage of the voltage controller. The CTL sensor cell was constructed by inserting the ceramic heater into a homemade quartz tube (length = 9 cm, inner diameter = 1 cm, outer diameter = 1.2 cm) with inlet and outlet. A miniature PTC vaporizing device (power: 60 W, working voltage: 24 V, length of central cylinder = 2.5 cm, diameter of central cylinder = 2 cm, length of cylinder joint = 1.5 cm, diameter of cylinder joint = 0.6 cm, customized from Dongsheng Electronic Heating and Appliance Factory, Taizhou, China) was coupled with the sensor cell. The vaporizing device was linked to another voltage controller (Power II). The liquid sample (5 μL) was injected into the vaporizing device, whereupon it was vaporized immediately, and then driven toward the sensor cell by a flow of air supplied by an air pump. A BPCL Ultra Weak Chemiluminescence Analyzer (Biophysics Institute of Chinese Academy of Science, China) equipped with a photomultiplier (PMT) was used to record the CTL intensity. The detection wavelengths could be selected over the range 350–550 nm by changing the optical filters.The data acquisition time for each signal point was set as 0.5 s, and the voltage for the photomultiplier tube was 850 V. The data was recorded with a computer and was further processed with OriginPro. The response of the n-propanol sensor is defined as
| S = I − N | (1) |
2.4 Instrumental analysis for mechanism study
To explore the possible reaction mechanism of n-propanol on the SrCO3/graphene composite, an Agilent 7890A GC-MS equipped with a HP-INNO Wax column (30 m, 0.25 mm inner diameter, and 0.25 μm-thick film) was used to analyze the products from the catalytic oxidation. The exhaust gas from the catalytic oxidation was collected in a sampling bag, and then 200 μL of the sample was injected into the instrument.3. Results and discussion
3.1 Characterization of the sensing material
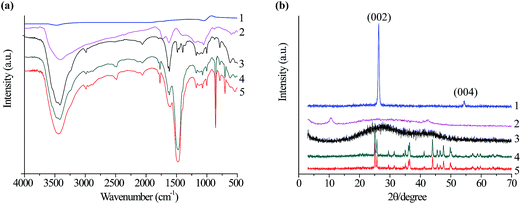
Fig. 2a shows the FT-IR spectra of pristine graphite powder, GO, graphene, SrCO3 and SrCO3/graphene. The spectra reveal that after graphite oxidation, the GO contains several functional groups, including OH (3432 cm−1), COOH (1724 cm−1), and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (1625 cm−1). Upon reduction of graphene oxide to graphene, the C
O (1625 cm−1). Upon reduction of graphene oxide to graphene, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O band disappears and new bands at 2988 and 2865 cm−1 appear, corresponding to the C–H stretch vibrations of the methylene group. Similar results were reported by Naebe et al.35 The band at 1474 cm−1 in the IR spectrum for SrCO3 corresponds to the asymmetric stretching mode of C–O bond. The sharp peaks at 857 and 702 cm−1 are in plane and out plane bending of CO32−, respectively. The graphene peaks are not visible in the IR spectrum for SrCO3/graphene, possibly because of the graphene groups are less active and its content in the SrCO3/graphene composite is low.
O band disappears and new bands at 2988 and 2865 cm−1 appear, corresponding to the C–H stretch vibrations of the methylene group. Similar results were reported by Naebe et al.35 The band at 1474 cm−1 in the IR spectrum for SrCO3 corresponds to the asymmetric stretching mode of C–O bond. The sharp peaks at 857 and 702 cm−1 are in plane and out plane bending of CO32−, respectively. The graphene peaks are not visible in the IR spectrum for SrCO3/graphene, possibly because of the graphene groups are less active and its content in the SrCO3/graphene composite is low.
 | ||
| Fig. 2 The FT-IR spectra (a) and XRD patterns (b) of different materials. Pristine graphite powder (line 1), GO (line 2), graphene (line 3), SrCO3 (line 4), SrCO3/graphene (line 5). | ||
Fig. 2b shows the XRD patterns of pristine graphite powder, GO, graphene, SrCO3 and SrCO3/graphene composite. A sharp, intense peak is observed at the (002) diffraction line (d-spacing of 0.35 nm at 26.35°) and a weak (004) diffraction peak at 54.6° is specific for graphite powder. GO shows a diffraction peak at 2θ of 10.6° corresponding to a d-spacing of 0.8 nm. The diffraction peak at about 10.6° of GO is weaker than that of pristine graphite at about 26.35°, which confirms the oxidation of pristine graphite to GO. Compared with pristine graphite, the intensity of the (002) peak of graphene decreases and broadened in its width. The XRD pattern of SrCO3 is consistent with orthorhombic SrCO3 (strontianite, JCPDS: 05-0418). The XRD peak of the SrCO3/graphene composite indicates that the intensity of the SrCO3 peak is low compared with the characteristic pure SrCO3 peaks because of the low content and low diffraction intensity of SrCO3, whereas the GO peaks disappear. Xu et al. reported that if regular stacks of graphite oxide or graphite are destroyed, for example, by exfoliation, then their diffraction peaks weaken or may even disappear.36 Therefore, the disappearance of the graphene peaks was attributed to exfoliation of GO sheets.
Fig. 3 shows TEM images of the graphene, SrCO3 and SrCO3/graphene composite. Fig. 3a shows a mixture of few-layer graphene with flake-like structures on top of the copper grid. The TEM micrograph of SrCO3 in Fig. 3b shows that SrCO3 forms orthogonal crystals, which is consistent with the XRD results. Fig. 3c shows that the SrCO3 was decorated with graphene, and was partially intercalated between two graphene sheets. The specific surface areas of SrCO3, graphene and SrCO3/graphene were measured by N2 (77.4 K) adsorption and desorption isotherms. The results show that the specific surface areas of SrCO3, graphene and SrCO3/graphene were 37, 786 and 104 m2 g−1, respectively. The specific surface area of the SrCO3/graphene composite was increased considerably compared with pure SrCO3.
3.2 Optimization
Fig. 4b shows the CTL intensity versus SrCO3 with different doping concentrations of graphene. The CTL intensity increased monotonically with increasing doping concentrations of graphene up to 12 wt%. SrCO3 doped with 12 wt% graphene exhibited the best response, and was chosen for further sensing characterization.
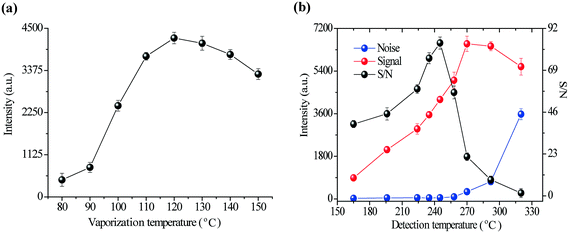
Fig. 5b shows the CTL intensity, background noise and S/N versus detection temperature at a fixed vaporization temperature of 120 °C. The CTL signal increased as the detection temperature increased and reached its maximum at 258 °C. However, the background noise, which mainly resulted from the thermal radiation of the heated sensing material, also increased with the temperature, which decreased S/N substantially at high temperatures. Therefore, a temperature of 245 °C was chosen as the optimum detection temperature because this was the temperature at which the S/N ratio reached its maximum.
Fig. 6b shows the change in the CTL intensity versus flow rate at 425 nm. The catalytic oxidation reaction of n-propanol was controlled by the diffusion rate below 260 mL min−1, which showed that the total reaction rate was controlled by the rate of the transfer of n-propanol from the gas phase to the catalyst surface. Thus, the CTL intensity was proportional to flow rate. However, the total reaction rate of n-propanol was limited by the oxidation rate of n-propanol on the catalyst surface when the flow rate was above 260 mL min−1, meaning that the CTL intensity was independent of the flow rate. Therefore, 260 mL min−1 was chosen as the optimal flow rate for the subsequent work.
3.3 Sensor performance
The sensor was stored at room temperature, and the effect of storage time on the stability was investigated by measuring the CTL intensity of n-propanol at a concentration of 10 mg L−1 every 24 h for 1 week. The sensor exhibited long-term stability with an RSD of 6.5% for seven replicate determinations during 1 week of periodic use.
3.4 Analytical figures of merit
Fig. 8 shows the CTL response versus the different concentrations of n-propanol under the optimized conditions. There was a linear relationship between the CTL intensity and n-propanol concentration from 0.2 to 32 mg L−1. The linear equation for the SrCO3/graphene-based n-propanol sensor was characterized by the equation I = 414.5C + 104.9 (correlation coefficient r = 0.9989), where I is the relative CTL intensity and C is the concentration of n-propanol. The LOD at an S/N of 3 was 0.08 mg L−1.3.5 Sample analysis
To evaluate the analytical applications of the sensor, samples spiked with known concentrations of n-propanol were prepared for recovery experiments. n-Propanol, benzaldehyde and β-phenylethanol are commonly used as flavoring agents in food manufacturing and n-propanol is commonly found in alcoholic beverages. Therefore, sample 1 contained equal concentrations of benzaldehyde (10 mg L−1) and β-phenylethanol (10 mg L−1), and sample 2 was Tsingtao beer (alcohol content: 2.5%). The two samples were spiked with n-propanol standards at concentrations of 1, 5 and 10 mg L−1 for recovery experiments. As shown in Table 1, the recoveries for n-propanol in different samples were 82.4–116.5% with RSDs of 5.7–10.4%, indicating the utility of the sensor for rapid detection of n-propanol in real samples.3.6 Possible mechanism
Based on widely accepted widely accepted theories about CL reactions, excited intermediates are probably formed during the catalytic oxidation of n-propanol. The luminescence could be produced when the excited intermediates decay to the ground state. To study the mechanism, GC-MS experiments were performed to identify the reaction products of the catalytic oxidation of n-propanol on SrCO3 and the SrCO3/graphene composite. As Fig. 9 shows, the catalytic oxidations of n-propanol on both SrCO3 and the SrCO3/graphene composite produced n-propanal, acetaldehyde and carbon dioxide, indicating that the enhanced CTL intensity does not result from new products, and n-propanol undergoes the same reaction path on SrCO3 and on SrCO3/graphene. The residual amounts of n-propanol, propionaldehyde and acetaldehyde on SrCO3/graphene were smaller than those on SrCO3, whereas the amount of carbon dioxide on SrCO3/graphene was higher than that on SrCO3. This result indicates that the catalytic oxidation on SrCO3/graphene is more efficient than that on pure SrCO3. The specific surface area of SrCO3/graphene was larger than that of pure SrCO3, which may explain the increased efficiency of catalytic oxidation for the SrCO3/graphene composite.The formation of propionaldehyde during catalytic oxidation may suggest that the initial attack of oxygen on n-propanol occurs mainly at the α-C–H and O–H bonds. The formation of copious amounts of carbon dioxide indicates that it may be the final product. Fig. 7b shows that propionaldehyde produced a strong CTL signal, and acetaldehyde produced a weak CTL signal on the SrCO3/graphene composite. It has been reported that acetaldehyde and carbon dioxide in excited states are the important luminous intermediates generated during the CTL reaction.40,41 Therefore, we propose that the oxidation of propionaldehyde and acetaldehyde during the oxidation of n-propanol are mainly responsible for the CTL emission. According to these results, the possible oxidation mechanism for n-propanol could be described by the following reactions: an oxygen molecule is adsorbed by the catalyst to form an activated oxygen molecule (reaction (2)); propionaldehyde is formed from the reaction of n-propanol with the oxygen (reaction (3)); propionaldehyde is oxidized to produce acetaldehyde and carbon dioxide, accompanied by a strong photoemission (reaction (4)); and acetaldehyde is oxidized to produce carbon dioxide, which generates a weak photoemission (reaction (5)).
 | (2) |
 | (3) |
 | (4) |
 | (5) |
Cullis et al. reported that the gaseous oxidation of n-propanol shows considerable similarity to that of ethanol, both of which initially form their corresponding aldehydes. The principal difference then between the oxidation of 2-propanol and the corresponding reaction of primary alcohols is that a ketone is formed instead of an aldehyde as the main initial reaction product.42 Cullis et al. stated that the α-C–H bond should be more reactive in n-propanol than in ethanol because the electron-repelling CH3 group is further from the point of attack. The relative reactivities of these alcohols toward oxygen are in the order n-propanol > ethanol > 2-propanol. The different reaction paths for n-propanol and 2-propanol, and the higher reactivity of n-propanol may result in the higher CTL signal for n-propanol than for ethanol and 2-propanol on the SrCO3/graphene composite (Fig. 7b). However, further work should be done to understand the mechanism of the CTL reaction of n-propanol on the SrCO3/graphene composite.
4. Conclusion
A SrCO3/graphene composite was synthesized for an n-propanol sensor. The effect of the graphene loading concentrations on the sensing properties of SrCO3 for n-propanol was investigated. We concluded that the graphene in the composite enhanced the sensitivity of the composite toward n-propanol, and a graphene content of 12 wt% was optimum for fabricating the n-propanol sensor. The sensor was coupled with a miniature vaporizing device and used to analyze n-propanol in liquid samples, which expanded the range of suitable samples. The detecting conditions of the sensor include vaporization temperature, detection temperature, wavelength, and flow rate for determining n-propanol were systematically optimized. The sensor was used to determine the amount of n-propanol in spiked samples. The recoveries were 82.4–116.5% with RSDs of 5.7–10.4%, respectively. Finally, the possible reaction mechanism behind the n-propanol on the SrCO3/graphene composite was investigated by GC-MS. The sensor is not limited to determining n-propanol in liquid samples; it also can be used for rapid sensing of gaseous n-propanol. Therefore, our work provides a selective, sensitive and convenient method for the rapid determination of n-propanol.Acknowledgements
This study was supported by the Science and Technology Foundation of the Guizhou Provincial Science and Technology Department (no. LH20147406), the Natural Science Foundation of the Guizhou Provincial Education Department (no. 2012070), and the Social Development Foundation of the Science and Technology Bureau of Qianxinan Prefecture (no. 201430).References
- K. Novoselov, A. K. Geim, S. Morozov, D. Jiang, M. K. I. Grigorieva, S. V. Dubonos and A. A. Firsov, Nature, 2005, 438, 97–200 CrossRef PubMed.
- H. Pei, J. Li, M. Lv, J. Y. Wang, J. M. Gao, J. X. Lu, Y. P. Li, Q. Huang, J. Hu and C. H. Fan, J. Am. Chem. Soc., 2012, 134, 13843–13849 CrossRef CAS PubMed.
- R. K. Paul, S. Badhulika, N. M. Saucedo and A. Mulchandani, Anal. Chem., 2012, 84, 8171–8178 CrossRef CAS PubMed.
- Y. Y. Su and Y. Lv, RSC Adv., 2014, 4, 29324–29339 RSC.
- D. Z. Zhang, J. Q. Liu, H. Y. Chang, A. M. Liu and B. K. Xia, RSC Adv., 2015, 5, 18666–18672 RSC.
- P. G. Su and S. L. Peng, Talanta, 2015, 132, 398–405 CrossRef CAS PubMed.
- L. Z. Yu, L. C. Zhang, H. J. Song, X. M. Jiang and Y. Lv, CrystEngComm, 2014, 16, 3331–3340 RSC.
- Y. X. Liu, X. C. Dong and P. Chen, Chem. Soc. Rev., 2012, 6, 2283–2307 RSC.
- F. Yavari and N. Koratkar, J. Phys. Chem. Lett., 2012, 3, 1746–1753 CrossRef CAS.
- W. J. Yuan and G. Q. Shi, J. Mater. Chem. A, 2013, 1, 10078–10091 CAS.
- G. Singh, A. Choudhary, D. Haranath, A. G. Joshi, N. Singh, S. Singh and R. Pasricha, Carbon, 2012, 50, 385–394 CrossRef CAS PubMed.
- F. Liu, X. F. Chu, Y. P. Dong, W. B. Zhang, W. S. Sun and L. M. Shen, Sens. Actuators, B, 2013, 188, 469–474 CrossRef CAS PubMed.
- Y. F. Zhu, J. J. Shi, Z. Y. Zhang, C. Zhang and X. R. Zhang, Anal. Chem., 2002, 74, 120–124 CrossRef CAS.
- T. Okabayashi, T. Fujimoto, I. Yamamoto, K. Utsunomiya, T. Wada, Y. Yamashita, N. Yamashita and M. Nakagawa, Sens. Actuators, B, 2000, 64, 54–58 CrossRef CAS.
- J. Z. Zheng, W. X. Zhang, J. Cao, X. H. Su, S. F. Li, S. R. Hu, S. X. Li and Z. M. Rao, RSC Adv., 2014, 4, 21644–21649 RSC.
- N. Na, S. C. Zhang, S. Wang and X. R. Zhang, J. Am. Chem. Soc., 2006, 128, 14420–14421 CrossRef CAS PubMed.
- Y. Wu, N. Na, S. C. Zhang, X. Wang, D. Liu and X. R. Zhang, Anal. Chem., 2009, 81, 961–966 CrossRef CAS PubMed.
- R. K. Zhang, Y. F. Hu and G. K. Li, Anal. Chem., 2014, 86, 6080–6087 CrossRef CAS PubMed.
- R. K. Zhang, X. A. Cao, Y. H. Liu and X. Y. Chang, Anal. Chem., 2013, 85, 3802–3806 CrossRef CAS PubMed.
- L. J. Zhang, W. Q. Rong, Y. C. Chen, C. Lu and L. X. Zhao, Sens. Actuators, B, 2014, 205, 82–87 CrossRef CAS PubMed.
- J. Y. Han, F. F. Han, J. Ouyang, L. X. He, Y. T. Zhang and N. Na, Nanoscale, 2014, 6, 3069–3072 RSC.
- L. Xu, H. J. Song, J. Hu, Y. Lv and K. L. Xu, Sens. Actuators, B, 2012, 169, 261–266 CrossRef CAS PubMed.
- W. F. Niu, H. Kong, H. Wang, Y. T. Zhang, S. C. Zhang and X. R. Zhang, Anal. Bioanal. Chem., 2012, 402, 389–395 CrossRef CAS PubMed.
- H. L. Zhang, L. C. Zhang, J. Hu, P. Y. Cai and Y. Lv, Talanta, 2010, 82, 733–738 CrossRef CAS PubMed.
- X. Y. Wan, H. J. Song, D. Zhao, L. C. Zhang and Y. Lv, Sens. Actuators, B, 2014, 201, 413–419 CrossRef CAS PubMed.
- P. Yang, X. N. Ye, C. W. Lau, Z. X. Li, X. Liu and J. Z. Lu, Anal. Chem., 2007, 79, 1425–1432 CrossRef CAS PubMed.
- Y. X. Chu, Q. C Zhang, Y. H. Li, Z. M. Xu and W. R. Long, Microchim. Acta, 2014, 181, 1–8 CrossRef.
- K. W. Zhou, Y. L. Cheng, H. W. Yang, C. X. Gu, Y. Xiao and M. H. Zhao, Sens. Actuators, B, 2014, 202, 721–726 CrossRef CAS PubMed.
- H. J. Song, L. C. Zhang, C. L. He, Y. Qu, Y. F. Tian and Y. Lv, J. Mater. Chem., 2011, 21, 5972–5977 RSC.
- Inert Reassessment-n-Propanol. WASHINGTON, D.C. 20460 office of prevention pesticides, and toxit substances, http://www.epa.gov/opprd001/inerts/propanol.pdf, accessed August 2005.
- D. W. Lachenmeier, S. Haupt and K. Schulz, Regul. Toxicol. Pharmacol., 2008, 50, 313–321 CrossRef CAS PubMed.
- New jersey department of health and senior services. Hazardous substance fact sheet, http://nj.gov/health/eoh/rtkweb/documents/fs/1605.pdf, accessed May 2005.
- B. Gulbakan, E. Yasun, M. I. Shukoor, Z. Zhu, M. X. You, X. H. Tan, H. Sanchez, D. H. Powell, H. J. Dai and W. H. Tan, J. Am. Chem. Soc., 2010, 132, 17408–17410 CrossRef CAS PubMed.
- V. K. Ponnusamy and J. F. Jen, J. Chromatogr. A, 2011, 1218, 6861–6868 CrossRef CAS PubMed.
- M. Naebe, J. Wang, A. Amini, H. Khayyam, N. Hameed, L. H. Li, Y. Chen and B. Fox, Sci. Rep., 2014, 4, 1–7 Search PubMed.
- C. Xu, X. Wang and J. W. Zhu, J. Phys. Chem. C, 2008, 112, 19841–19845 CAS.
- C. C. Wu, X. A. Cao, Q. Wen, Z. H. Wang, Q. Q. Gao and H. C. Zhu, Talanta, 2009, 5, 1223–1227 CrossRef PubMed.
- Y. Tao, X. A. Cao, Y. Peng and Y. H. Liu, Sens. Actuators, B, 2010, 1, 292–297 CrossRef PubMed.
- Y. H. Liu, F. Tang, C. J. Kang and X. A. Cao, Luminescence, 2012, 4, 274–278 CrossRef PubMed.
- Z. Y. Zhang, K. Xu, W. R. G. Baeyens and X. R. Zhang, Anal. Chim. Acta, 2005, 1, 145–152 Search PubMed.
- X. Wang, N. Na, S. C. Zhang, Y. Y. Wu and X. R. Zhang, J. Am. Chem. Soc., 2007, 129, 6062–6063 Search PubMed.
- C. F. Cullis and E. J. Newitt, Proc. R. Soc. London, Ser. A, 1960, 1290, 402–412 CrossRef.
| This journal is © The Royal Society of Chemistry 2015 |