 Open Access Article
Open Access ArticleBODIPY-phenylacetylene macrocycle motifs for enhanced light-harvesting and energy transfer applications†
M.
Yousaf
a,
A. J.
Lough
b,
E.
Schott
c and
B. D.
Koivisto
a
aDepartment of Chemistry and Biology, Ryerson University, 350 Victoria St, Toronto, Canada. E-mail: bryan.koivisto@ryerson.ca
bDepartment of Chemistry, University of Toronto, Toronto, Canada
cLaboratorio de Bionanotecnología, Universidad Bernardo O'Higgins, General Gana 1702, Santiago, Chile
First published on 26th June 2015
Abstract
A supramolecular BODIPY molecule functionalized with a phenylacetylene macrocycle is reported. Light absorption by the macrocycle results in energy transfer to the BODIPY core as evidenced by fluorescence studies. This supramolecular motif demonstrates an effective strategy for panchromatic light-harvesting.
Nature's light-harvesting capabilities depend on supramolecular cyclic chromophores for capturing, transferring and converting solar energy.1 In photosynthetic systems, rigid protein frameworks facilitate rapid energy transfer from highly-conjugated antenna complexes to the reaction centre, and based on this inspiration mimicking antenna scaffolds has become a popular pursuit towards solar energy production.2 To this end, there has been considerable interest in developing intensely absorbing rigid dyestuffs capable of mimicking this natural behaviour. One such family of chromophores are the shape-persistent phenylacetylene macrocycles.3 Owing to their intense light absorption, these extended π-structures have been explored in a number of optoelectronic and light-harvesting applications,4 including two-photon absorption. Two-photon absorption is an attractive feature for light-harvesting dye design because it represents a potential form of up-conversion.5 Likewise, BODIPY (4,4-difluoro-4-bora-3a,4a-diaza-s-indacenes) dyes have also been explored in a number of material applications, owing to their tunable, intense absorption and sharp emission peaks exhibiting high quantum yields.6
As part of our continued interest in organic based dyes for dye-sensitized solar cell (DSSC) applications7 we were interested in combining the phenylacetylene macrocycle and our existing BODIPY motifs8 to create and explore a family of dyes that could be used in light-harvesting applications. Our hypothesis being that upon absorption of light, the phenylacetylene macrocycle would transfer energy to the BODIPY core, thereby increasing the effective absorption envelope. To this end, we report herein the synthesis and physicochemical properties of an orthogonal phenylacetylene macrocycle BODIPY, 7 (Scheme 1).
 | ||
| Scheme 1 Synthesis of orthogonal BODIPY-phenylacetylene macrocycle, 7, and benchmark macrocycle 8. Synthesis of 1, 4, and 8 are included in the ESI.† | ||
Intense investigation into phenylacetylenes has resulted in robust synthetic methodologies and a variety of shapes and architectures.9 Inspired by a phenylacetylene molecular turnstile,10 and using similar synthetic strategies, our original attempt sought to make the hexagonal phenylacetylene macrocycle starting from BODIPY derivative 1. Owing to facile and competing Glaser coupling (unoptimized), pentaphenyl derivative 7 was the only isolated product.
After extensive characterisation efforts, the identity of 7 was confirmed using X-ray crystallography (Fig. 1; single crystals were grown from saturated dichloromethane (DCM)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane (1
hexane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solutions). In the solid state, the dihedral angle between the BODIPY and attached phenyl ring is 118°, suggesting that the two chromophores are not truly orthogonal. However, in solution 1H-NMR equivalent environments throughout the macrocycle and BODIPY suggest that there is a significant amount of rotational freedom for the BODIPY within the cavity, but not enough for it to adopt a coplanar conformation within the macrocycle (vide infra, DFT).
1) solutions). In the solid state, the dihedral angle between the BODIPY and attached phenyl ring is 118°, suggesting that the two chromophores are not truly orthogonal. However, in solution 1H-NMR equivalent environments throughout the macrocycle and BODIPY suggest that there is a significant amount of rotational freedom for the BODIPY within the cavity, but not enough for it to adopt a coplanar conformation within the macrocycle (vide infra, DFT).
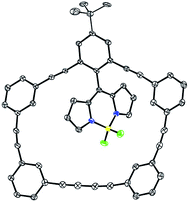 | ||
| Fig. 1 X-ray crystal structure of 7 (ORTEP, 30% thermal ellipsoids). Hydrogen atoms and solvent molecules have been removed for clarity. | ||
Absorption data collected in DCM solutions and time-dependent density functional theory (TDDFT) assigned transitions (labelled according to orbitals in Fig. 3) are provided in Fig. 2. The absorption envelope of macrocycle 7 is effectively the superimposition of starting BODIPY derivative 1 and benchmark macrocycle 8. Similar to most BODIPY derivatives, building block 1, has a strong absorption at 515 nm and a hyposochromic shoulder; and the remainder of the spectrum is relatively featureless. In 7, these BODIPY absorptions are slightly blue-shifted (510 nm), and a series of strong absorptions owing to the macrocycle are present below 350 nm.
 | ||
| Fig. 2 Absorption spectra of BODIPY-phenylacetylene macrocycle, 7 and benchmark BODIPY 1, in DCM. Dominant optical transitions have been assigned using TD-DFT.‡ | ||
DFT calculations (B3LYP, 6-311G*)‡ of the frontier molecular orbitals (FMOs) are presented in Fig. 3. The minimized DFT structure possesses a slightly bowl-shaped phenylacetylene macrocycle, consistent with the observed structure in the solid state. Unlike the solid-state structure, the optimized structure sees the BODIPY essentially orthogonal to the attached phenyl ring with a dihedral angle of 89.97°. In addition, most FMOs predominantly have electron density on the macrocycle, except the HOMO−2 and HOMO−1 that have significant contributions localised on the BODIPY portion of the molecule, and the LUMO is localised exclusively on the BODIPY.
TDDFT calculations were performed to elucidate the nature of the observed absorption behaviour. Consistent with the assignments in Fig. 2, the largest optical transition (292 nm) was assigned as the HOMO to LUMO+2. This absorption is localized on the macrocycle and these intense π–π* absorptions arise from optical transitions that possess significant electron density overlap between the ground and excited state.
All optical transitions below 350 nm are dominated by strong π–π* absorptions localised predominantly on the phenylacetylene macrocycle. Absorptions centred at 510 nm, are due to optical transitions that are primarily localized on the BODIPY (HOMO−1 to LUMO), and are consistent with previously reported.8
Emission data collected in DCM for 7 is presented in Fig. 4 (quantum yields are presented in Table S1,† and an excitation spectrum is included in Fig. S2†). When comparing the emission of 7 to benchmark 8, distinct differences emerge with respect to the fluorophore. Upon excitation of 8 at 287 nm (Fig. S1†), emission from the macrocycle results in a Stokes shift of 46 nm. Conversely, when 7 is excited at 292 nm, emission appears to originate from the BODIPY portion of the molecule resulting in a ‘Stokes-like’ shift of 236 nm. Irrespective of excitation wavelength (292, 487 or 510 nm) an emission from BODIPY and proportional to the absorption extinction coefficient, is observed at 528 nm. This is consistent with Kasha's rule where one would expect fluorescence from the lowest excited state, (i.e. the LUMO which is located predominantly on the BODIPY portion of the molecule).
 | ||
| Fig. 4 UV-vis absorption and fluorescence emission spectra (in DCM) for BODIPY-phenylacetylene macrocycle, 7. Emission spectra were acquired by exciting at 292, 487, and 510 nm, respectively. | ||
These results are consistent with other previously reported oligomeric phenylacetylene architectures, where exciton accumulation was observed in π-conjugated wires sheathed by phenylacetylene macrocycles – despite the wire not being conjugated to the macrocycle.11 More recently, energy transfer has been observed in coordination driven structures with BODIPYs installed on the periphery.12 Using non-covalent host–guest complexes, it was shown that highly efficient energy transfer is observed from a guest to the BODIPY containing host.13 Combining these popular phenylacetylene macrocycle and BODIPY motifs, our work demonstrates that effective supramolecular energy transfer can occur between these intensely absorbing chromophores. Moreover, this motif should be suitable as a π-spacer for DSSC applications as the orthogonal nature of our structure has ideal energy transfer form the macrocycle to the BODIPY core of the molecule and could be modified for panchromatic absorption.
Conclusions
In conclusion, we have synthesized a supramolecular BODIPY, covalently bound and threaded within a phenylacetylene macrocycle. To our knowledge, this is the first supramolecular example that combines both motifs. Aided by the physicochemical characterization of benchmark 8, absorption by the macrocycle (7) results in energy transfer to the BODIPY core, resulting in characteristic BODIPY emission behaviour. With these promising results in hand, we are currently developing derivatives more suitable for DSSC applications by installing donor and acceptor motifs onto the BODIPY. In addition, we are also attempting to rigidify the BODIPY within the macrocycle to enforce an orthogonal arrangement. Furthermore, we are also designing phenylacetylene macrocycles that have extended conjugation in hopes of shifting the phenylacetylene macrocycle absorption to longer and more relevant absorption wavelengths, ideally within the visible spectrum.Notes and references
- G. D. Scholes, G. R. Fleming, A. Olaya-Castro and R. van Grondelle, Nat. Chem., 2011, 3, 763 CrossRef CAS PubMed.
- M. W. Cooke and G. S. Hanan, Chem. Soc. Rev., 2007, 36, 1466 RSC.
- J. S. Moore, Acc. Chem. Res., 1997, 30, 402 CrossRef CAS.
- S. Campagna, F. Puntoriero, F. Nastasi, G. Bergamini and V. Balzani, Photochemistry and Photophysics of Coordination Compounds I, 2007, vol. 280, p. 117 Search PubMed.
- B. A. Reinhardt, L. L. Brott, S. J. Clarson, A. G. Dillard, J. C. Bhatt, R. Kannan, L. X. Yuan, G. S. He and P. N. Prasad, Chem. Mater., 1998, 10, 1863 CrossRef CAS.
- A. Loudet and K. Burgess, Chem. Rev., 2007, 107, 4891 CrossRef CAS PubMed.
- C. Bonnier, D. D. Machin, O. K. Abdi, K. C. Robson and B. D. Koivisto, Org. Biomol. Chem., 2013, 11, 7011 CAS.
- C. Bonnier, D. D. Machin, O. Abdi and B. D. Koivisto, Org. Biomol. Chem., 2013, 11, 3756 CAS.
- S. Hoger, Angew. Chem., Int. Ed., 2005, 44, 3806 CrossRef PubMed.
- T. C. Bedard and J. S. Moore, J. Am. Chem. Soc., 1995, 117, 10662 CrossRef CAS.
- K. Becker, P. G. Lagoudakis, G. Gaefke, S. Hoeger and J. M. Lupton, Angew. Chem., Int. Ed., 2007, 46, 3450 CrossRef CAS PubMed.
- A. Kaloudi-Chantzea, N. Karakostas, F. Pitterl, C. P. Raptopoulou, N. Glezos and G. Pistolis, Chem. Commun., 2012, 48, 12213 RSC.
- N. Karakostas, I. M. Mavridis, K. Seintis, M. Fakis, E. N. Koini, I. D. Petsalakis and G. Pistolis, Chem. Commun., 2014, 50, 1362 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Full characterisation and synthetic procedures are included. CCDC 1057238. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra08592f |
| ‡ These calculations were enabled in part by the support provided by WestGrid (http://www.westgrid.ca) and Compute Canada Calcul Canada (http://www.computecanada.ca), using Gaussian 09, Revision D.01 (see ESI† for further details). |
| This journal is © The Royal Society of Chemistry 2015 |

