A double core–shell modification of bulk TiO2 microspheres into porous N-doped-graphene carbon nanoflakes/N-doped TiO2 microspheres for lithium-ion battery anodes†
Balasubramaniyan Rajagopalan,
Eun-Suok Oh,
Won Mook Choi and
Jin Suk Chung*
School of Chemical Engineering, University of Ulsan, Namgu, Daehakro 93, Ulsan 680-749, Republic of Korea. E-mail: jschung@mail.ulsan.ac.kr; Fax: +82 52 259 1689; Tel: +82 52 259 2249
First published on 22nd April 2015
Abstract
In this study, we modified bulk TiO2 microspheres—using a template-aided, double core–shell modification with N-doped carbon (NC) and N-doped graphene (NG)—for the purpose of forming porous N-doped graphene carbon nanoflakes/N-doped TiO2 (NG–NC/NTiO2) microspheres. The effects of surface modification on the properties of the TiO2 microspheres and the resultant electrochemical performance in a lithium-ion-battery (LIB) anode were thoroughly investigated. The double core–shell modified nanocomposite exhibited a specific capacity of 74 mA h g−1 at a 10 C rate, which was much higher than the capacities of TiO2, carbon/TiO2, and core–shell NC/NTiO2 nanocomposites at rates of 0.2, 1, and 5 C, respectively. The RGO of the double core–shell NC/NTiO2 nanocomposite provided an effective buffering effect for the TiO2 microsphere, resulting in a much lower initial specific-capacity loss of 19.6%, on the 200th cycle, in comparison with the 41.8% loss of the core–shell NC/NTiO2 nanocomposite at the same cyclic stage. Such excellent performances from the TiO2 microspheres with the double core–shell assembly in the LIB anode were attributed to a significant reduction of charge transfer resistance (Rct) and maintenance of electrode stability.
Introduction
As an anode material of lithium-ion batteries (LIB), the abundant, low-cost, and eco-friendly TiO2 has recently received a large amount of attention due to its higher operating voltage (higher than Li by over 1.5 V) and the benefit of its sound structural stability during Li-ion insertion/extraction processes.1–4 Poor electrical conductivity and ionic diffusivity, however, are still major drawbacks hampering the commercialization of bulk micro-sized TiO2.5–8 To address these issues, attempts at reducing the particle size to the nanometer (nm) level, increasing the porosity, doping with metal atoms, and compositing with carbon-based materials have all occurred;5,7,9,10 in terms of nano-sized particles, though, the resultant agglomeration and increase of irreversible capacity loss also hinder the potential for practical application.11–15 The transport-path length of the lithium ions and charge transport across the electrode/electrolyte boundaries were, however, enhanced when porous TiO2 was introduced to LIBs.12,16–22Also during the recent time period, carbon-based materials such as carbon, reduced graphene oxide (RGO), and carbon nanotubes have been extensively studied in relation to TiO2, as the use of carbonaceous materials increases electrical conductivity while also reducing volume expansion;23–26 furthermore, the monetary cost of carbon-based materials for doping TiO2 is lower than that of metal atoms. Glucose or the carbonization of titanium glycolate are the most commonly examined facilitative options for the incorporation of carbon into TiO2 structures.1,27,28 The uniform coating of the carbon has, however, posed a considerable challenge for the carbon-based metal-oxide electrodes.29,30 For bulk TiO2 from 1–10 μm in size, Liu et al. applied the carbon-incorporation technique to improve the electrochemical performance in LIBs.1 However, even after carbon incorporation, the resultant carbon/TiO2 microsphere exhibited a significantly low specific capacity that was lower than 100 mA h g−1 at a 0.2 C rate. Alternatively, precisely controlled carbon loading that performs excellently in LIBs can be obtained through a surface coating of resorcinol–formaldehyde polymer and subsequent calcinations, whereby the polymer coating prevents the destruction of the pore structure while increasing the crystallinity of the TiO2 during calcination.29 Another interesting carbon-based material is two-dimensional (2D) graphene, which is widely used in hybrids containing metal oxides due to its outstanding electrical and mechanical properties.28,31–33 Thus, the inclusion of RGO not only increases electrical conductivity, but also acts as a buffer to reduce metal-oxide volume changes during Li-ion intercalation and de-intercalation reactions.6 In addition, the doping of carbon or RGO with N has been demonstrated as an effective method for improving the electrochemical performances of carbon-based nanocomposites.3,34 The extra lone pair of electrons provided by the doped N atoms forms a complex with the metal oxide and aids the RGO or carbon hybridization process.35 Recently, nitridation of TiO2, which generates electrically conductive TiN on the TiO2, has been gaining more attention in relation to LIBs.36–38 It is possible that N doping produces more defects on the RGO, which would facilitate the efficient diffusion of Li ions between the RGO and metal oxides.13,23
Very recently, core–shell assemblies of metal oxides such as TiO2, Fe2O3, TiO2–carbon/MnO2, TiO2–Sn/carbon, TiO2–MnO2/MnO2, and TiO2/SnO2 were applied to LIB anodes to enhance the capacity and cyclic stability of the electrodes;39–44 however, the surface of the metal core–shell significantly retards the Li ions' direct access into the inner core.44 While electrical conductivity and electrochemical performance were significantly increased for carbon/TiO2 core shells,1,29 overloading or the use of a dense layer is not recommended for Li batteries due to the consequent hindrance of Li-ion mobility into the internal areas of the metal oxides.30,45 To address these problems, we designed a double core–shell enhancement of the bulk-TiO2-microsphere structure using vertically decorated NC as the primary core–shell—without forming a dense layer—to increase electrical conductivity, and a secondary core–shell of defective NG to not only provide electrical conductivity, but also a buffer effect for the reduction of volume expansion.
Herein, we propose a method for improving the electrochemical performance of bulk TiO2 microspheres. The aforementioned double core–shell modification involved the decoration of NC and NG, while the preparation of the nanocomposites was carried out in four steps: The first step involved the decoration of the TiO2 microspheres with Mn3O4; the second and third steps included the polymerization of aniline into polyaniline (PANI) using Mn3O4 as an oxidative template, and the preparation of GO–PANI/TiO2 using the electrostatic interactions of GO and PANI/TiO2; and the final step was the carbonization of the GO–PANI/TiO2. During the carbonization of PANI, the bulk TiO2 was changed into a porous structure, and parts of the TiO2 were turned into electrically conductive TiO2–TiN. The NG–NC/NTiO2 nanocomposite was finally obtained; furthermore, the PANI itself acts as an N dopant with carbon, RGO, and TiO2, so the use of additional doping reagents was not required. The advantages of this method are as follows: (1) prevention of increased TiO2 crystallinity during calcination; (2) maintenance of the stability of the material due to the buffer effect of RGO; (3) the charge transport to the interior of the bulk TiO2 through the TiO2–TiN is easy; and (4) increased electrical conductivity from the contact between the TiO2–TiN and the double core–shell comprised of NC and NG.
Experimental
Materials and methods
Expandable graphite (Grade 1721) was purchased from Asbury Carbon, USA. Concentrated sulfuric acid (H2SO4), potassium permanganate (KMnO4), hydrochloric acid (HCl), aniline (ANI), acetonitrile, and hydrogen peroxide (H2O2) were purchased from Samchun Chemical, Korea. Ethylene glycol (EG) was purchased from OCI, Korea. Manganese acetate tetrahydrate was purchased from Junsei, Japan. Hydrazine monohydrate, sodium lauryl sulfate (SLS), and TiCl4 were purchased from Sigma Aldrich, USA. All chemicals were used as they were received without further purification.Preparation of titanium glycolate microspheres
Titanium glycolate microspheres were derived from the solvothermal reactions of TiCl4 in a mixture of EG and acetonitrile. Typically, 4 g of TiCl4 was slowly dispersed in 50 ml of EG and acetonitrile at a volume ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. The resultant clear solution was transferred to a 100 ml Teflon-lined, stainless-steel autoclave and hydrothermally heated at 180 °C for 24 h. The final product was washed with deionized water several times and then dried in an oven at 80 °C for 12 h.
3. The resultant clear solution was transferred to a 100 ml Teflon-lined, stainless-steel autoclave and hydrothermally heated at 180 °C for 24 h. The final product was washed with deionized water several times and then dried in an oven at 80 °C for 12 h.
Preparation of Mn3O4-decorated TiO2 microspheres
Typically, 1.2 g of titanium glycolate microspheres was dispersed in 50 ml of deionized water using mechanical stirring for 15 min, followed by ultra-sonication for 10 min. About 1.2 g of SLS was then added to the titanium glycolate–water suspension using stirring of around 200 rpm. We then stirred 20 g of manganese acetate tetrahydrate into the suspension until complete dispersion occurred. The final precursor solution was transferred into a 100 ml Teflon-lined stainless-steel autoclave and heated at 160 °C for 12 h. During the solvothermal reaction, the titanium glycolate microspheres hydrolyzed with the water and changed into TiO2 microspheres. The impurities were removed from the final Mn3O4-decorated TiO2 microspheres using water and ethanol. The final product was dried in an oven at 60 °C for 12 h, and named “Mn3O4/TiO2”.Preparation of PANI/TiO2
The oxidative polymerization of ANI was carried out using Mn3O4 as a template. Typically, 1 g of Mn3O4/TiO2 and 1 ml of ANI was suspended in 100 ml of deionized water. The solution was then sonicated for 5 min before being placed into an ice bath using gentle stirring. Next, concentric HCl was added to the Mn3O4/TiO2 suspension until a final concentration of 1 M was reached. After polymerization for 5 h, the black-colored PANI/TiO2 nanocomposite was obtained. The nanocomposite was then washed several times with water and ethanol to remove the residual ANI, and was dried at 60 °C for 12 h.Preparation of double core–shell RTNG–NC/NTiO2, TNG–NC/NTiO2, and core–shell NC/NTiO2 nanocomposites
GO was prepared from expandable graphite in accordance with the modified Hummers method.46 About 25 mg of GO (5 mg ml−1) suspension and a predetermined amount of PANI/TiO2 were placed into a mortar-pestle. The nanocomposite suspension was then gently grained (about 2–5 min) to obtain a homogeneous GO–PANI/TiO2 nanocomposite, which was finally dried in an oven at 80 °C for 12 h. To reduce the GO, the dried nanocomposite powder was placed into 50 ml of water, prior to its reaction with hydrazine (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, GO/hydrazine) at 85 °C for 6 h in an oil bath without stirring. For N doping, the RGO–PANI/TiO2, GO–PANI/TiO2, and PANI/TiO2 were calcined at 700 °C for 4 h, with a 10 °C min−1 temperature increase in an N2 atmosphere, to obtain RTNG–NC/TiO2, TNG–NC/TiO2, and NC/TiO2, respectively. The control sample of TiO2 was prepared firstly through the solvothermal reaction of titanium glycolate powder at 160 °C for 12 h, followed by the application of all of the aforementioned calcination-reaction conditions except the use of an N2 atmosphere, which was replaced by an air atmosphere. For comparison, a carbon/TiO2 nanocomposite was prepared by directly calcining the titanium glycolate microspheres in an N2 atmosphere.
10, GO/hydrazine) at 85 °C for 6 h in an oil bath without stirring. For N doping, the RGO–PANI/TiO2, GO–PANI/TiO2, and PANI/TiO2 were calcined at 700 °C for 4 h, with a 10 °C min−1 temperature increase in an N2 atmosphere, to obtain RTNG–NC/TiO2, TNG–NC/TiO2, and NC/TiO2, respectively. The control sample of TiO2 was prepared firstly through the solvothermal reaction of titanium glycolate powder at 160 °C for 12 h, followed by the application of all of the aforementioned calcination-reaction conditions except the use of an N2 atmosphere, which was replaced by an air atmosphere. For comparison, a carbon/TiO2 nanocomposite was prepared by directly calcining the titanium glycolate microspheres in an N2 atmosphere.
Characterization
Field-emission scanning electron microscopy (FESEM, JEOL, JSM-6500F) was used to characterize the surface morphologies of the TiO2, RTNG–NC/NTiO2, TNG–NC/NTiO2, and NC/NTiO2 nanocomposites. Field-emission transmission electron microscopy (FETEM; JEM 2100 F, JEOL) was used to analyze the internal structures of TiO2 and its nanocomposites. Thermal gravimetric analysis (TGA; Q50, TA) was used to characterize thermal properties. Raman spectrometry (Thermo scientific, DXR) was performed with a 633 nm incident laser for characterization of the TiO2 and graphene. A high-power X-ray diffractometer (Rigaku, Japan), set at 2θ between 5 and 80°, was used to perform X-ray diffraction (XRD) analyses, and a Thermo Fisher instrument with Al Kα radiation (energy range: 200 eV to 3 keV) was used to perform X-ray photoelectron spectroscopy (XPS). A BET (Brunauer–Emmett–Teller) surface-area analyzer (Micromeritics, ASAP 2020) measured surface area and porosity. A Won A Tech (WBCS 3000) battery tester characterized electrochemical charge/discharge and cyclic voltammetry, and, lastly, a BioLogic Science Instrument measured the electrochemical impedance spectra (EIS).Electrochemical characterizations
The electrochemical reactions of the TiO2, RTNG–NC/NTiO2, TNG–NC/NTiO2, and NC/NTiO2 nanocomposites were performed using CR2016-type coin cells. To prepare the working electrodes, 80 wt% active material, 15 wt% super P carbon black, and 5 wt% of polyvinylidene fluoride dispersed in N-methyl-2-pyrrolidone were cast onto a copper current collector. The electrodes were fabricated into a coin cell after vacuum drying at 80 °C for 12 h. The battery consisted of TiO2 for a working electrode; Li metal for a counter/reference electrode; porous polypropylene film for a separator; and 1 M of LiPF6 in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) mixture of ethylene carbonate, dimethyl carbonate; and ethyl methyl carbonate for an electrolyte. The charge/discharge characteristics of the TiO2, RTNG–NC/NTiO2, TNG–NC/NTiO2, and NC/NTiO2 nanocomposites were measured at a rate in the range of 0.2–10 C. The specific capacity was calculated based on a theoretical TiO2 capacity of 168 mA h g−1 at a 1 C rate. The EIS was performed at frequencies from 100 kHz to 0.01 Hz.
1 (v/v) mixture of ethylene carbonate, dimethyl carbonate; and ethyl methyl carbonate for an electrolyte. The charge/discharge characteristics of the TiO2, RTNG–NC/NTiO2, TNG–NC/NTiO2, and NC/NTiO2 nanocomposites were measured at a rate in the range of 0.2–10 C. The specific capacity was calculated based on a theoretical TiO2 capacity of 168 mA h g−1 at a 1 C rate. The EIS was performed at frequencies from 100 kHz to 0.01 Hz.
Results and discussion
The fabrication of the double core–shell of NC and NG sought to reduce volume change during the electrochemical tests while also increasing the electrical conductivity of the TiO2. As shown in Fig. 1, the NC/NTiO2 and NG–NC/NTiO2 were prepared through a Mn3O4, template-assisted oxidative polymerization of aniline into PANI, followed by precipitation with GO and calcination. During the solvothermal reaction of titanium glycolate for the Mn3O4 decoration, the titanium glycolate hydrolyzed with water to produce Mn3O4/TiO2. The oxidative polymerization of aniline for PANI/TiO2 in the presence of HCl replaced the Mn3O4 with PANI via the removal of soluble Mn2+ ions. The template method was applied to PANI to reduce the aggregation/separation of PANI from the TiO2 surface, as well as to reduce the formation of bulk-layered PANI. GO coating was then carried out through electrostatic interactions between the amino groups of the PANI with hydroxyl and the carboxylic groups of the GO. The subsequent reduction of GO with hydrazine and the following calcination were carried out to obtain RTNG–NC/NTiO2, while the NG and TiO2–TiN were formed as a result of the carbonization of PANI at the interlayer. The main advantages of this method are that the formation of a dense carbon layer is avoided, and additional N dopant was not required for the doping of the RGO, carbon, and TiO2.The FE-SEM images in Fig. 2a–d show the 0D microspheres of titanium glycolate with a sphere diameter of 5–10 μm. The uniform morphology of the microspheres with a layer-like morphology on the titanium glycolate surfaces can be observed in Fig. 2a–c; further, the higher magnification of the titanium glycolate shows the crumpled form with a densely layered surface (Fig. 2d). The Mn3O4-decorated TiO2 microspheres are shown in Fig. 3a and b; the low and high magnification images of the Mn3O4/TiO2 microspheres show that 2D-like Mn3O4 nanoflakes with an average size of 200 nm were uniformly arranged vertically and continuously on the TiO2 microspheres. Interestingly, after the replacement of the template with PANI (Fig. 3c and d) and the subsequent calcination to NC (Fig. 3e and f), morphological features similar to those of the Mn3O4 nanoflakes were observed. The continuously decorated NC and the free inter-particle spaces can significantly increase the corresponding electrical conductivity and ionic diffusion. Only a trace amount of the Mn3O4 remained after washing, which was confirmed by SEM-EDXA (energy dispersive X-ray analysis) spectra (Fig. S1, ESI†).
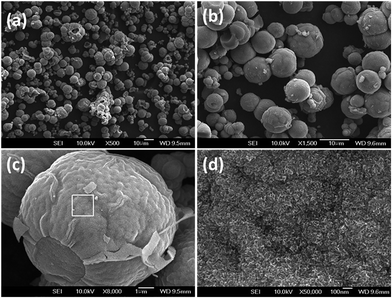 | ||
| Fig. 2 FE-SEM images of titanium glycolate microspheres at various magnifications (a–d). The (d) image is the highlighted magnification represented by the square in (c). | ||
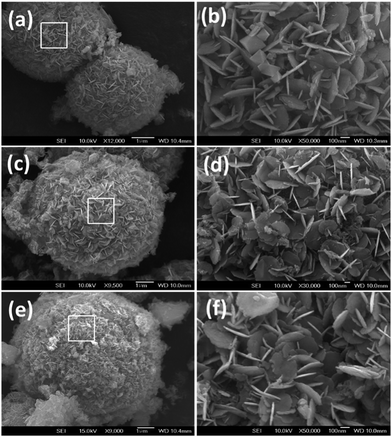 | ||
| Fig. 3 FE-SEM images of (a) Mn3O4/TiO2, (c) PANI/TiO2, and (e) NC/NTiO2 nanocomposites. The (b), (d), and (f) images show higher magnifications of (a), (c), and (e), respectively. | ||
To gain a clear understanding of the TiO2 surface modification and surface changes in this study, the TiO2, carbon/TiO2, and NC/TiO2–air FE-SEM images were analyzed, as shown in Fig. S2 (ESI†). The direct carbonization of titanium glycolate changed the crumpled surface into densely grown, extremely small nanoparticles; however, regarding TiO2, bulk particles 50–100 nm in size emerged after the titanium–glycolate solvothermal reactions. It is noteworthy that pores were not clearly evident on the surfaces of both the TiO2 and carbon/TiO2 microspheres. Interestingly, in the case of the NC/NTiO2–air nanocomposite, mesopores were clearly seen between nano-sized particles 10–20 nm in size, in contrast to both the TiO2 and carbon/TiO2 nanocomposites. We therefore inferred that the gas formed by the carbonization of PANI diffused into the internal areas of the TiO2, modifying the bulk structure into that of a porous nature. The nano-sized NC/NTiO2 particles are well-suited for LIBs, as they can induce ionic diffusion through the inter-particle mesopores.
Fig. 4a and b present the double core–shell structure of the RTNG–NC/NTiO2 microsphere, wherein it is clear that the core–shell NC/NTiO2 microsphere is uniformly covered with a second core–shell of NG sheets; further, the higher magnification shows that the NG sheets were closely attached to the NC/NTiO2. The very clear depiction of the NC of the RTNG–NC/NTiO2 microsphere, shown in Fig. S3 (ESI†), was caused by the stacking of two or more NG sheets on the NC/NTiO2 microspheres. To analyze the internal structures of the nanocomposites, FETEM analysis was carried out, as shown in Fig. 4c–f. In Fig. 4c, the dark color of the TiO2 microsphere indicates the bulk characteristics, whereas the brighter areas observed on the core–shell NC/NTiO2 microspheres are indicative of the porous nature of TiO2 and the flower-like surface is NC (Fig. 4d). Interestingly, surface NG layers still appeared in the FETEM images taken after the ultra-sonication of the double core–shell RTNG–NC/NTiO2 (Fig. 4e and f), indicating a stronger bond between the NG and NC. The inset shown in Fig. 4f is the higher magnification of the NG sheets of the RTNG–NC/NTiO2 microspheres and clearly shows the stacked layer consisting of two or more NG sheets.
 | ||
| Fig. 4 FE-SEM images of (a and b) RTNG–NC/NTiO2 nanocomposite, and TEM images of (c) pristine TiO2, (d) NC/NTiO2, and (e and f) RTNG–NC/NTiO2. | ||
XRD was used for an analysis of the crystallographic structure of the nanocomposites. As shown in Fig. 5a, the XRD pattern of the Mn3O4/TiO2 nanocomposite presented the characteristic peaks for both anatase TiO2 (JCPDS no. 21-1272) and Mn3O4 (JCPDS no. 24-0734). After the oxidative polymerization and conversion of Mn3O4 to PANI, the XRD peaks for Mn3O4 disappeared, leaving only major peaks for the anatase TiO2. The uncalcined nanocomposites revealed broader, shorter peaks, representing a lower crystallinity. In Fig. 5b, the sharp diffraction peaks corresponding to the anatase phase were due to the higher crystallinity of the TiO2 microspheres.13,47 The presence of GO and PANI significantly prevented the increase of crystallinity during calcination, resulting in slightly broader peaks for the surface-modified nanocomposites compared to pristine TiO2.29 Interestingly, the surface-modified nanocomposites showed some residual peaks, corresponding with rutile TiO2 (JCPDS no. 77-0443) and TiO2 (B) (JCPDS no. 46-1238), along with the major diffraction peaks of anatase TiO2. A new peak for the surface-modified nanocomposites in comparison to TiO2 or carbon/TiO2 at around 35° is due to the formation of a Ti2O3 phase (JCPDS no. 01-074-2277), which occurred during the surface modification of TiO2. The characteristic peak of RGO or carbon at 26° was difficult to identify for the surface-modified nanocomposites due to a weak intensity or an overlap with the major diffraction peak of TiO2.6,37 The Scherrer equation was applied to determine the 101 (2θ = 25.3°) major intensity peak of TiO2 and the surface-modified nanocomposites for the purpose of calculating the average particle size.48,49 The particle sizes of the TiO2 in the nanocomposites were estimated to be 47.6 nm (TiO2), 9.9 nm (carbon/TiO2), 11.4 nm (NC/NTiO2), 17.3 nm (TNG–NC/NTiO2), and 17.0 nm (RTNG–NC/NTiO2) (Table S1, ESI†). The decreasing particle size of the surface-modified nanocomposites was ascribed to the conversion of the bulk TiO2 microspheres into porous microspheres comprised of many nano-sized particles during the carbonization of PANI. It is interesting to note that the calculated particle sizes of the nanocomposites are highly compatible with the SEM images shown in Fig. S2 (ESI†).
 | ||
| Fig. 5 XRD nanocomposite analysis of (a) Mn3O4/TiO2 and PANI/TiO2. (b) TiO2 and surface-modified TiO2. | ||
Next, TGA was carried out to measure the carbon and RGO contents in the TiO2 nanocomposites, the results of which are shown in Fig. S4a (ESI†). The weight loss of the nanocomposites observed below 200 °C was due to the evaporation of the adsorbed moisture and water. The functional groups and carbon on the PANI, NG, and NC decomposed as the temperature increased to 800 °C. After complete decomposition, the total weight losses of the carbon or carbon/RGO in the PANI/TiO2, NC/NTiO2, TNG–NC/NTiO2, and RTNG–NC/NTiO2 nanocomposites were 15%, 2.7%, 5.6%, and 5.5%, respectively. The graphene contents of the nanocomposites were measured at about 0% for NC/NTiO2, 2.9% for TNG–NC/NTiO2, and 2.8% for RTNG–NC/NTiO2. The presence of NC was similar for all of the surface modified nanocomposites at about 2.7%. Interestingly, the TGA curve of the RTNG–NC/NTiO2 nanocomposite showed a low thermal decomposition at around 500–600 °C compared with the TNG–NC/NTiO2 nanocomposite due to the higher thermal stability of the hydrazine-reduced GO. Fig. S4b (ESI†) shows the TGA of the carbon/TiO2 and TiO2–N2 nanocomposites, for which they exhibited weight losses corresponding to carbon loadings of 7.8% and 0.05%, respectively. The low carbon loading (0.05%) of the TiO2–N2 nanocomposite was representative of the complete oxidation of titanium glycolate into TiO2.
The XPS analysis of the NC/NTiO2 nanocomposite is presented in Fig. 6a–c. As shown in Fig. 6a, the survey scan spectra of the NC/NTiO2 nanocomposite confirmed the presence of the elements carbon, N, Ti, and O. To check the N doping of the carbon and TiO2, the N1s spectra was analysed, as shown in Fig. 6b. The deconvoluted N1s spectrum of the NC/NTiO2 nanocomposite presented four main peaks centered at 398.2 eV (pyridinic-N), 399.6 eV (pyrrolinic-N and O–Ti–N), 400.8 eV (graphitic-N), and 403.8 eV (pyridinic-NOx), and represented the doping of N into carbon and TiO2.23,37,48 It should be noted that N doping partially changed the TiO2 into TiO2–TiN, which was observed by the formation of a peak corresponding to TiN at 396.2 eV.37,50 We believe that this TiO2–TiN was formed in the internal areas and the surface-NC interfaces through a diffusion of gas into the internal areas of the TiO2 microspheres during the calcination of PANI. The elemental analysis of the NC/NTiO2 revealed that the nanocomposite contained about 3.1% N. To further confirm the presence of TiO2–TiN, the Ti2p spectrum was taken and displayed in Fig. 6c. The Ti2p spectrum presented a broader peak centered at 457.4 eV, along with the main peaks of Ti2p3/2–1/2, which was due to the formation of metallic TiN on the TiO2.50 Similar characteristic features were also observed in the RTNG–NC/NTiO2 nanocomposite (Fig. S5, ESI†), indicating that N doping occurred on both the carbon and RGO sheets. As metallic TiN is electrically conductive, it was expected to increase the charge transports in the LIB electrode.
 | ||
| Fig. 6 XPS analysis of (a) survey-scan spectra, (b) N1s spectra, and (c) Ti2p spectra of the NC/NTiO2 nanocomposite. | ||
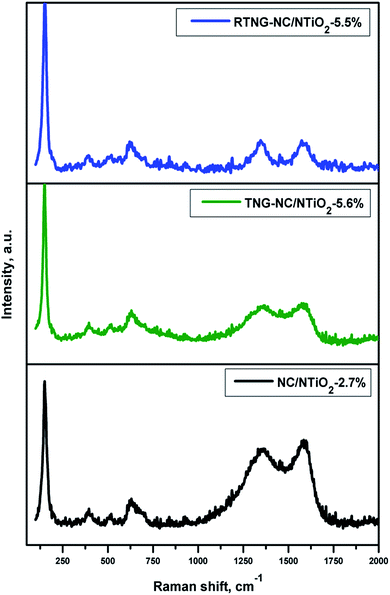
Fig. 7 presents the Raman spectra of the NC/NTiO2, TNG–NC/NTiO2, and RTNG–NC/NTiO2 nanocomposites. Regarding the anatase TiO2, the Raman spectra of the nanocomposites displayed peaks at 148, 394, 511, and 630 cm−1 for Eg, B1g, A1g, and Eg, respectively.11,23,51 Additional peaks were observed for the nanocomposite near 1350 and 1580 cm−1, corresponding to the D and G peaks for the NC and NG–NC, respectively. The D band was associated with the defects and the disordered nature of the RGO sheets, while the G band was a result of the first-order scattering of the E2g mode of the sp2 carbon.50,51 The ratio of ID/IG was used to measure the degree of disorder originating from the defects of the nanocomposites. As shown in Fig. 7, the ID/IG ratios of the NC/NTiO2, TNG–NC/NTiO2, and RTNG–NC/NTiO2 nanocomposites were measured as 0.9, 0.96, and 1.1, respectively. The increasing ID/IG ratios of the TNG–NC/NTiO2 and RTNG–NC/NTiO2 nanocomposites were due to increased defects caused by N doping and reduction on the RGO sheets.50,52,53 Moreover, the degree of reduction was also analyzed with the D shift value of the GO, with a lower D shift indicating a higher reduction.54,55 The D band value of the RTNG–NC/NTiO2 was 1346 cm−1, which was lower than that of the TNG–NC/NTiO2 (1355 cm−1), representing the higher reduction of the hydrazine-reduced GO sheets.
The specific surface areas and pore structures of the nanocomposites were analyzed using a nitrogen isothermal adsorption technique. As shown in Fig. 8a, the nitrogen adsorption–desorption isotherms of the C/TiO2 nanocomposite provided an isothermal curve that is very similar to type-II (non-porous) with a very small hysteresis loop, indicating the non-porous nature of the carbon/TiO2 nanocomposite. Interestingly, the isothermal plots for all of the nanocomposites prior to the solvothermal modification followed the type-IV isotherm with a large hysteresis loop, which is characteristic of a mesoporous composition. The specific surface area of the NC/NTiO2 nanocomposite was 45.6 m2 g−1, which was much higher than that of the TiO2 nanocomposite (8.75 m2 g−1), and was due to the presence of NC and the surface modification of TiO2 via the carbonization of PANI. Interestingly, the TNG surface coating considerably decreased the specific surface area to 34.7 m2 g−1, but the specific surface area of the RTNG–NC/NTiO2 (59.1 m2 g−1) nanocomposite increased significantly in comparison with the NC/NTiO2 and TNG–NC/NTiO2 nanocomposites due to the presence of RGO. It should be noted that the specific surface area of the carbon/TiO2 nanocomposite was 82.3 m2 g−1, which is a higher value that can be explained by the increasing surface area that corresponds with the tiny nano-sized particles on the surface (Fig. S2, ESI†). However, the pore-size distribution measured from the desorption isotherm using the Barrett–Joyner–Halenda (BJH) method exhibited a very low value of 3.3 nm (Fig. 8b), indicating that the carbonization of the titanium glycolate did not substantially increase the pore size of the adjacent TiO2 particles. Interestingly, the pore-size distribution of the surface-modified TiO2 microspheres exhibited an average of more than 7 nm (Fig. 8b). Large mesopores in the range of 7–31.5 nm and a pore volume of 0.137 cm3 g−1 were observed for the RTNG–NC/NTiO2 nanocomposite, with the latter value higher than the volume of the TNG–NC/NTiO2 nanocomposite, which displayed pore sizes of 14.1 to 35.8 nm and a pore volume of 0.105 cm3 g−1. The NC/NTiO2 nanocomposite also presented large mesopores and a higher pore volume, but the latter was lower than that of RTNG–NC/NTiO2. The significantly lower surface properties observed for TNG–NC/NTiO2 might be due to the poor porous characteristics of TGO on the NC/NTiO2 nanocomposite. The distinct porous nature observed for the RTNG–NC/NTiO2 nanocomposite was due to the RGO and the formation of large numbers of micropores on the RGO, as well as in the TiO2, through the gas released from PANI during carbonization. A detailed investigation of the pore sizes and pore volumes of the nanocomposites is shown in Table S2 (ESI†).
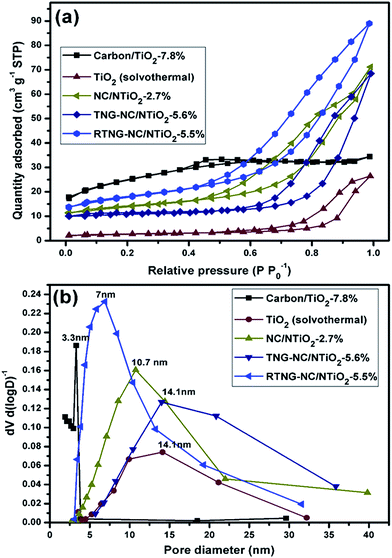 | ||
| Fig. 8 (a) Nitrogen adsorption–desorption isotherms of the TiO2, carbon/TiO2, NC/NTiO2, TNG–NC/NTiO2, and RTNG–NC/NTiO2 nanocomposites; (b) pore-size distribution of the nanocomposites of (a). | ||
The TiO2 and carbon/TiO2 nanocomposites, the core–shell structure of the NC/TiO2 nanocomposite, and the double core–shell structures of the TNG–NC/NTiO2 and RTNG–NC/NTiO2 nanocomposites were employed in the fabrication of an LIB electrode to distinguish the influences of the non-core–shell, the core–shell, and the double core–shell structures of the TiO2 microspheres on the specific capacity and electrochemical stability. After using the following conditions, we strongly believe that the double core–shell structures of TNG–NC/NTiO2 and RTNG–NC/NTiO2 would offer excellent LIB performances: the three different but continuous electrical-conducting layers of TiO2–TiN, NC, and NG for higher electrical conductivity; the porous nanoparticles of modified TiO2 with defective NG for efficient Li-ion transport; and the buffering effect of NC–NG for material stability.
The electrochemical-rate capability tests for TiO2 and its modified nanocomposites were performed at different C rates, from 0.2 C to 10 C, and the results are shown in Fig. 9a.
 | ||
| Fig. 9 (a) Rate capability test of the nanocomposites at various C rates from 0.2 C to 10 C. (b) EIS analysis of the TiO2, NC/NTiO2-2.7%, TNG-NC/NTiO2-5.6%, and RTNG-NC/NTiO2-5.5%. | ||
Remarkably, the pristine TiO2 exhibited a very low specific capacity of 30 mA h g−1, even at a 0.2 C rate, and the specific capacity displayed a significant decrease down to 5 mA h g−1 when the C rate was increased to 10 C. The lower rate capability of TiO2 was highly compatible with the characteristic features of the bulk TiO2 microspheres.1,56,57 It should be noted that the carbon/TiO2 prepared with the formal method facilitated an increase of the specific capacity at 0.2 C to 110 mA h g−1, but that this also decreased rapidly to 10 mA h g−1 at 10 C; this poor rate capability can be explained by the non-porous characteristics of the carbon/TiO2 (Fig. 8). On the other hand, the rate capabilities of the modified TiO2 nanocomposites including the core–shell NC/NTiO2 and the double core–shells of TNG–NC/NTiO2 and RTNG–NC/NTiO2, were highly superior to both TiO2 and carbon/TiO2. Their capacities at 10 C were higher than 40 mA h g−1, while they ranged from 165 to 180 mA h g−1 at 0.2 C. To summarize, the rate capability of the nanocomposites increased in the order of TiO2 < carbon/TiO2 < core–shell NC/NTiO2 < double core–shell TNG–NC/NTiO2 < double core–shell RTNG–NC/NTiO2, which can be explained by the distinct porous characteristics shown in Fig. 8 and by the electrical conductivity.
It is widely accepted that an improvement of electrical conduction increases the specific capacity of TiO2 nanocomposites.23,35,51 Even though NC/NTiO2 and its nanocomposites do not contain conductive carbons in the interior of the TiO2 microspheres, electrically conductive TiO2–TiN (Fig. 6) exists in the internal areas; gas diffusion from the calcination of PANI results in its presence up to a pore volume of 0.1–0.137 cm3 g−1 (Table S2, ESI†). Moreover, surface-NC further increases the electrical conductivity of the NTiO2, which can explain the higher electrochemical performances displayed by NC/NTiO2 and its nanocomposites when compared with carbon/TiO2-7.8%.
For an enhanced understanding, EIS of the modified TiO2 nanocomposites were performed and compared with the pristine TiO2, as displayed in Fig. 9b. The inset of the equivalent circuit model shows the solution resistance (Rs), charge transfer resistance (Rct), Warburg impedance (Rw), and double-layer capacitor (Cdl) of the impedance spectra. The size of the semicircle, which indicates the Rct of the electrochemical reactions,23,50 appeared in the order of TiO2 > NC/NTiO2 > TNG–NC/NTiO2 > RTNG–NC/NTiO2, which is the exact opposite of the order of the rate capability in Fig. 9a. Generally, Rct is closely related to electrical conduction, so that increases of electrical conductivity through an N-doping and RGO-induced modification of TiO2 contributes to a decrease in the Rct, ultimately enhancing the rate capability of TiO2.
The charge/discharge cycles of the TiO2, NC/NTiO2-2.7%, TNG–NC/NTiO2-5.6%, and RTNG–NC/NTiO2-5.5% nanocomposites, as they pertain to the C rates shown in Fig. 9a, are presented in Fig. 10a–d. Due to the poor electrical conductivity of bulk TiO2 microspheres, the first discharge capacity was as low as 35 mA h g−1. The surface modification via the core–shell and the double core–shell of NC and NG, as well as TiO2–TiN, significantly increased electrical conductivity and porosity, resulting in higher specific capacities in the range of 220 to 235 mA h g−1 for the surface-modified nanocomposites shown in Fig. 10b–d. The longer discharge plateau observed at 1.74 V for RTNG–NC/NTiO2-5.5% is attributed to the higher electrical conductivity of the N-doped RGO. The presence of RGO significantly reduced the irreversible capacity caused by the irreversible reactions between the functional groups of the unreduced GO sheets and lithium ions, leading to a higher initial coulombic efficiency of the RTNG–NC/NTiO2-5.5% sample in comparison with the TNG–NC/NTiO2-5.6% sample. The polarization of the potential plateaus of the nanocomposites decreased in the order of TiO2 > NC/NTiO2 > TNG–NC/NTiO2 > RTNG–NC/NTiO2, and was especially the case at high current densities due to the differences in electrical conductivity.50,51
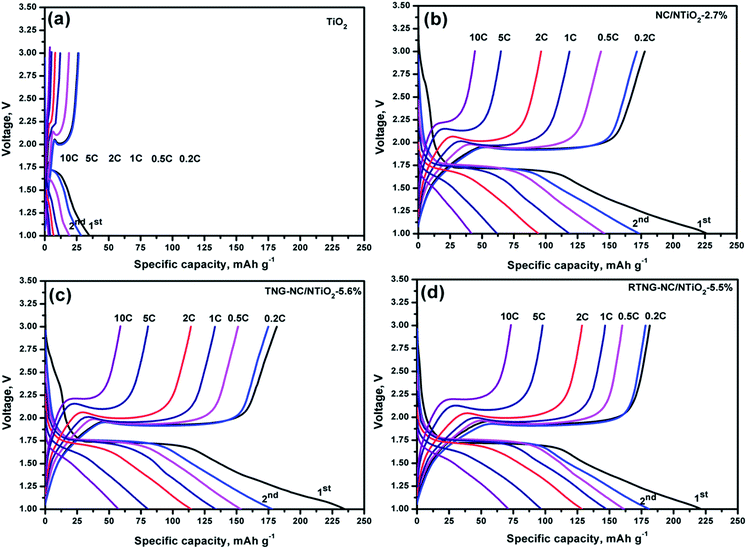 | ||
| Fig. 10 First charge/discharge cycles of TiO2, NC/NTiO2-2.7%, TNG–NC/NTiO2-5.6%, and RTNG–NC/NTiO2-5.5% at various C rates. | ||
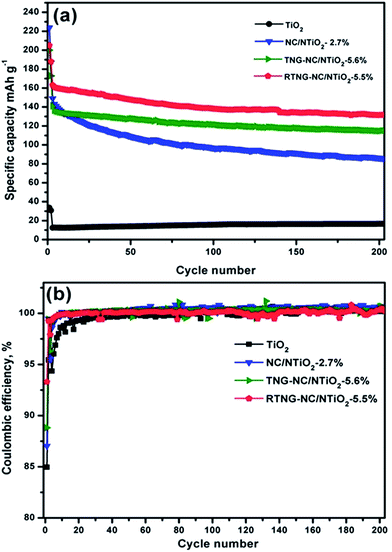
The cyclic test results of the samples performed at 0.2 C for the first 2 cycles, followed by 1 C for the next 200 cycles, are exhibited in Fig. 11a. As expected from the rate capability results in Fig. 9a, the double core–shell structures of the TNG–NC/NTiO2 and RTNG–NC/NTiO2 nanocomposites showed excellent cyclic-capacity retention compared with the core–shell NC/NTiO2 nanocomposite. At the 200th cycle, the TNG–NC/NTiO2 and RTNG–NC/NTiO2 nanocomposites lost only 18.51% and 19.6%, respectively, from their initial capacities, whereas NC/NTiO2 lost 41.8%; such marked differences are responsible for the superior volume stability of the TiO2 inside the double core–shells. The outer-most graphene layer acts as a buffer for the prevention of volume change during the cycles, while the porous structure of TiO2 and the active sites in the defective NG increase the transport of Li ions.23 The lower cyclic capacity and better cyclic stability of the TNG–NC/NTiO2 nanocomposite compared with the RTNG–NC/NTiO2 nanocomposite might be due to the strong hybridization (Fig. 1) between the functional groups of the TGO and NC, which can help maintain the structure of the electrode during the cyclic process. The reduction of GO, however, causes a loss of this hybridization, thereby slightly decreasing the stability of the material. As shown in Fig. 11b, the coulombic efficiencies of the modified TiO2 nanocomposites reached nearly 100% on the 10th cycle, while the pristine TiO2 microspheres reached 98.5%, demonstrating the effective reversibility of the modified electrodes.
In summary, the excellent specific capacity, rate capability, and cycle stability of the surface-modified nanocomposites compared with the bulk TiO2 microspheres were due to the following: (1) the improvement of the porous characteristics of TiO2 by gas diffusion during the calcination of PANI; (2) the formation of an electrically conductive network of TiO2–TiN, NC, and NG; and (3) the buffering effect of the double core–shell assembly of NC–NG.
Conclusions
We developed a double core–shell method for the surface modification of bulk TiO2 microspheres into porous and highly conductive TiO2 microspheres containing NC, NG, and TiO2–TiN by following the oxidative-template method. Our findings revealed that the calcination of GO–PANI/TiO2 or RGO–PANI/TiO2 for the decoration of NC and NG not only increased the electrical conductivity, but also increased the porosity and formation of TiO2–TiN as a result of the diffusion of gas from PANI into the internal areas of the TiO2 microspheres. Owing to an effective buffering effect created by the synergistic effects of the electrically conductive RGO, NC, defective NG, and TiO2–TiN in the TiO2 microspheres, as well as the higher electrical conductivity of the double core–shell, the RTNG–NC/NTiO2-5.5% exhibited an excellent specific capacity, rate capability, and cyclic stability in comparison with the TiO2, carbon/TiO2-7.8%, NC/NTiO2-2.7%, and TNG–NC/NTiO2-5.6% nanocomposites. This surface-modification strategy is potentially more effective than existing strategies, and can be extended to any kind of material to increase electrical conductivity and improve the porosity of nanocomposites.Acknowledgements
This research was supported by the Basic Science Research Program of the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science and Technology (2011-0022485).Notes and references
- Z. Liu, J. Liu, J. Liu, L. Wang, G. Zhang and X. Sun, Phys. Chem. Chem. Phys., 2014, 16, 8808–8811 RSC.
- S.-T. Myung, N. Takahashi, S. Komaba, C. S. Yoon, Y.-K. Sun, K. Amine and H. Yashiro, Adv. Funct. Mater., 2011, 21, 3231–3241 CrossRef CAS PubMed.
- L. Tan, C. Cao, H. Yang, B. Wang and L. Li, Mater. Lett., 2013, 109, 195–198 CrossRef CAS PubMed.
- Z. Hong, M. Wei, T. Lan and G. Cao, Nano Energy, 2012, 1, 466–471 CrossRef CAS PubMed.
- Y. Ma, G. Ji, B. Ding and J. Y. Lee, J. Mater. Chem., 2012, 22, 24380–24385 RSC.
- X. Yan, Y. Li, F. Du, K. Zhu, Y. Zhang, A. Su, G. Chen and Y. Wei, Nanoscale, 2014, 6, 4108–4116 RSC.
- X. Su, Q. L. Wu, X. Zhan, J. Wu, S. Wei and Z. Guo, J. Mater. Sci., 2012, 47, 2519–2534 CrossRef CAS.
- C. Jiang, M. Wei, Z. Qi, T. Kudo, I. Honma and H. Zhou, J. Power Sources, 2007, 166, 239–243 CrossRef CAS PubMed.
- J. W. Kang, D. H. Kim, V. Mathew, J. S. Lim, J. H. Gim and J. Kim, J. Electrochem. Soc., 2011, 158, A59–A62 CrossRef CAS PubMed.
- J.-H. Jeong, D.-W. Jung, E. W. Shin and E.-S. Oh, J. Alloys Compd., 2014, 604, 226–232 CrossRef CAS PubMed.
- N. Li, G. Liu, C. Zhen, F. Li, L. Zhang and H. M. Cheng, Adv. Funct. Mater., 2011, 21, 1717–1722 CrossRef CAS PubMed.
- J. Jin, S.-Z. Huang, J. Liu, Y. Li, D.-S. Chen, H.-E. Wang, Y. Yu, L.-H. Chena and B.-L. Su, J. Mater. Chem. A, 2014, 2, 9699–9708 CAS.
- D. Li, D. Shi, Z. Liu, H. Liu and Z. Guo, J. Nanopart. Res., 2013, 15, 1674–1683 CrossRef.
- J. Wang, Y. Zhou, Y. Hu, R. O'Hayre and Z. Shao, J. Phys. Chem. C, 2011, 115, 2529–2536 CAS.
- P. G. Bruce, B. Scrosati and J. M. Tarascon, Angew. Chem., Int. Ed., 2008, 47, 2930–2946 CrossRef CAS PubMed.
- H. E. Wang, H. Cheng, C. Liu, X. Chen, Q. Jiang, Z. Lu, Y. Y. Li, C. Chung, W. Zhang and J. A. Zapien, J. Power Sources, 2011, 196, 6394–6399 CrossRef CAS PubMed.
- H. Wang, Z. Lu, L. Xi, R. Ma, C. Wang, J. Zapien and I. Bello, ACS Appl. Mater. Interfaces, 2012, 4, 1608–1613 CAS.
- H. Liu, Z. Bi, X.-G. Sun, R. R. Unocic, M. P. Paranthaman, S. Dai and G. M. Brown, Adv. Mater., 2011, 23, 3450–3454 CrossRef CAS PubMed.
- M. Alvaro, C. Aprile, M. Benitez, E. Carbonell and H. Garcia, J. Phys. Chem. B, 2006, 110, 6661–6665 CrossRef CAS PubMed.
- M. C. Carbajo, E. Enciso and M. Torralvo, Colloids Surf., A, 2007, 293, 72–79 CrossRef CAS PubMed.
- H. Shibata, T. Ogura, T. Mukai, T. Ohkubo, H. Sakai and M. Abe, J. Am. Chem. Soc., 2005, 127, 16396–16397 CrossRef CAS PubMed.
- D. H. Chen, F. Z. Huang, Y. B. Cheng and R. A. Caruso, Adv. Mater., 2009, 21, 2206–2210 CrossRef CAS PubMed.
- X. Jiang, X. Yang, Y. Zhu, H. Jiang, Y. Yao, P. Zhao and C. Li, J. Mater. Chem. A, 2014, 2, 11124–11133 CAS.
- Y. Xiao, C. Hu and M. Cao, Chem.–Asian J., 2014, 9, 351–356 CrossRef CAS PubMed.
- T. Xia, W. Zhang, Z. Wang, Y. Zhang, X. Song, J. Murowchick, V. Battaglia, G. Liu and X. Chen, Nano Energy, 2014, 6, 109–118 CrossRef CAS PubMed.
- Z. Sun, X. Huang, M. Muhler, W. Schuhmann and E. Ventosa, Chem. Commun., 2014, 50, 5506–5509 RSC.
- Q. Li, B. Liu, Y. Li, R. Liu, X. Li, D. Li, S. Yu, D. Liu, P. Wang, B. Li, B. Zou, T. Cui and G. Zou, J. Alloys Compd., 2009, 471, 477–480 CrossRef CAS PubMed.
- Y. Ren, J. Zhang, Y. Liu, H. Li, H. Wei, B. Li and X. Wang, ACS Appl. Mater. Interfaces, 2012, 4, 4776–4780 CAS.
- W. Wang, Q. Sa, J. Chen, Y. Wang, H. Jung and Y. Yin, ACS Appl. Mater. Interfaces, 2013, 5, 6478–6483 CAS.
- L. Tan, L. Pan, C. Cao, B. Wang and L. Li, J. Power Sources, 2014, 253, 193–200 CrossRef CAS PubMed.
- Y. Luo, J. Luo, W. Zhou, X. Qi, H. Zhang, D. Y. W. Yu, C. M. Li, H. J. Fan and T. Yu, J. Mater. Chem. A, 2013, 1, 273–281 CAS.
- Y. Sun, X. Hu, W. Luo, J. Shu and Y. Huang, J. Mater. Chem. A, 2013, 1, 4468–4474 CAS.
- V. H. Pham, T. T. Dang, T. V. Cuong, S. H. Hur, B. S. Kong, E. J. Kim and J. S. Chung, Korean J. Chem. Eng., 2012, 29, 680–685 CrossRef CAS PubMed.
- L. Tan, L. Pan, C. Cao, B. Wang and L. Li, J. Power Sources, 2014, 253, 193–200 CrossRef CAS PubMed.
- D. Cai, D. Li, S. Wang, X. Zhu, W. Yang, S. Zhang and H. Wang, J. Alloys Compd., 2013, 561, 54–58 CrossRef CAS PubMed.
- M.-S. Balogun, C. Li, Y. Zeng, M. Yu, Q. Wu, M. Wu, X. Lu and Y. Tong, J. Power Sources, 2014, 272, 946–953 CrossRef CAS PubMed.
- Y. Li, Z. Wang and X. J. Lv, J. Mater. Chem. A, 2014, 2, 15473–15479 CAS.
- H. Han, T. Song, J.-Y. Bae, L. F. Nazar, H. Kim and U. Paik, Energy Environ. Sci., 2011, 4, 4532–4536 CAS.
- H. Ren, R. Yu, J. Wang, Q. Jin, M. Yang, D. Mao, D. Kisailus, H. Zhao and D. Wang, Nano Lett., 2014, 14, 6679–6684 CrossRef CAS PubMed.
- S. Xu, C. M. Hessel, H. Ren, R. Yu, Q. Jin, M. Yang, H. Zhaoc and D. Wang, Energy Environ. Sci., 2014, 7, 632–637 CAS.
- J.-Y. Liao, D. Higgins, G. Lui, V. Chabot, X. Xiao and Z. Chen, Nano Lett., 2013, 13, 5467–5473 CrossRef CAS PubMed.
- J.-Y. Liao and A. Manthiram, Adv. Energy Mater., 2014, 4, 1400403–1400410 Search PubMed.
- X. Li, Y. Chen, H. Yao, X. Zhou, J. Yang, H. Huang, Y.-W. Mai and L. Zhou, RSC Adv., 2014, 4, 39906–39911 RSC.
- C. Zhu, X. Xia, J. Liu, Z. Fan, D. Chao, H. Zhang and H. J. Fan, Nano Energy, 2014, 4, 105–112 CrossRef CAS PubMed.
- Z. Zhu, F. Cheng and J. Chen, J. Mater. Chem. A, 2013, 1, 9484–9490 CAS.
- V. H. Pham, T. V. Cuong, S. H. Hur, E. Oh, E. J. Kim, E. W. Shin and J. S. Chung, J. Mater. Chem., 2011, 21, 3371–3377 RSC.
- Y. Qiu, K. Yan, S. Yang, L. Jin, H. Deng and W. Li, ACS Nano, 2010, 4, 6515–6526 CrossRef CAS PubMed.
- G. Qin, X. Zhang and C. Wang, J. Mater. Chem. A, 2014, 2, 12449–12458 CAS.
- H. E. Wang, J. Jin, Y. Cai, J.-M. Xu, D.-S. Chen, X.-F. Zheng, Z. Deng, Y. Li, I. Bello and B. L. Su, J. Colloid Interface Sci., 2014, 417, 144–151 CrossRef CAS PubMed.
- B. Rajagopalan, E. S. Oh and J. S. Chung, J. Power Sources, 2015, 275, 702–711 CrossRef CAS PubMed.
- J. Wang, L. Shen, H. Li, X. Wang, P. Nie, B. Ding, G. Xu, H. Dou and X. Zhang, Electrochim. Acta, 2014, 133, 209–216 CrossRef CAS PubMed.
- Z. Zhu, F. Cheng and J. Chen, J. Mater. Chem. A, 2013, 1, 9484–9490 CAS.
- H. Li, L. Shen, K. Yin, J. Ji, J. Wang, X. Wang and X. Zhang, J. Mater. Chem. A, 2013, 1, 7270–7276 CAS.
- B. Rajagopalan and J. S. Chung, Nanoscale Res. Lett., 2014, 9, 535–544 CrossRef PubMed.
- Z. Wen, X. Wang, S. Mao, Z. Bo, H. Kim, S. Cui, G. Lu, X. Feng and J. Chen, Adv. Mater., 2012, 24, 5610–5616 CrossRef CAS PubMed.
- X. Liu, Q. Sun, F. Liu, A. B. Djurisic, A. M. C. NG, M. Xie, T. Wood, J. A. Zapien, C. Liao and K. Shih, Turk. J. Phys., 2014, 38, 442–449 CrossRef CAS.
- A. K. Rai, L. T. Anh, J. Gim, V. Mathew, J. Kang, B. J. Paul, J. Song and J. Kim, Electrochim. Acta, 2013, 90, 112–118 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: EDAX, FE-SEM, TGA, XRD, BET, XPS, and Raman. See DOI: 10.1039/c5ra06573a |
| This journal is © The Royal Society of Chemistry 2015 |