Organic template-free synthesis of SAPO-34 molecular sieve membranes for CO2–CH4 separation
Hua Shi*
State Key Laboratory of Chemical Engineering, School of Chemical Engineering and Technology, Tianjin University, Weijin Road, Tianjin 300072, China. E-mail: shihua926@yahoo.com; Fax: +86 22 86986109; Tel: +86 22 86986109
First published on 23rd April 2015
Abstract
Continuous SAPO-34 molecular sieve membranes have been rapidly synthesized on porous α-Al2O3 supports by secondary growth in the absence of organic templates under microwave-assisted heating. The thus obtained SAPO-34 membranes exhibit CO2–CH4 separation selectivity as high as 256, with CO2 permeance higher than 1.68 × 10−6 mol m−2 s−1 Pa−1 at 295 K for a pressure drop of 0.14 MPa.
At present, selective separation of CO2 from a gas stream has attracted much interest from researchers because of its environmental significance and air purification for minimization of its effect on global warming.1 In particular, CO2 separation from CH4 is important in natural gas transportation and usage because CO2 reduces the energy content of natural gas, and it is acidic and corrosive in the presence of water.2 The technologies widely used for carbon dioxide removal are amine absorption and polymer membrane separation. However, the former is complex and costly,3 and the latter suffers from membrane plasticization and a decrease in its separation ability due to the high partial pressure of carbon dioxide.4 Compared with traditional polymer membranes, molecular sieve membranes exhibit promising application in purifying methane from CO2–CH4 mixture due to their high efficiency. Molecular sieve membranes can be potentially applied to separation and catalysis owning to their uniform pore size, good erosion resistance, and superior thermal, mechanical, and chemical stability at high pressure of CO2.5,6
Molecular sieve membranes used to gas separation and pervaporation have three types of pores: small,7–16 medium17,18 and large.19,20 The medium and large pores are much bigger than those of CO2 (0.33 nm kinetic diameter) and CH4 (0.38 nm), so the separation selectivity of these membranes is low due to competitive adsorption.17 In contrast, small-pore molecular sieve membranes such as molecular sieve T7,8 (0.36 × 0.51 nm pore diameter), DDR9 (0.36 × 0.44 nm), MOFs,10 mixed-matrix11,12 and SAPO-34 (ref. 13–16) (0.38 nm) have high CO2–CH4 selectivities due to a combination of differences in diffusivity and competitive adsorption because they are similar in pore size to CH4 (0.38 nm) but larger than CO2 (0.33 nm).
Up to now, molecular sieve membranes are failures to realize commercialized applications worldwide due to their high cost, unsatisfactory performances, environmental pollutions and difficulties in scaling up.21 The selectivity performances for molecular sieve membranes are affected by pinholes and cracks which almost led by removing template via high-temperature calcination. The existence of defects in molecular sieve membranes can be avoided if they are synthesized in the absence of organic templates. In typical hydrothermal synthesis of SAPO-34 molecular sieve membranes, the costly tetraethylammonium hydroxide (TEAOH) is usually used as the template. Therefore, the organic template-free syntheses of SAPO-34 molecular sieve membranes not only improve the selectivity performances but also reduce the cost of preparation remarkably.
The organic template-free preparation of molecular sieve membranes has been a rapidly growing research area due to reducing the cost significantly and avoiding the environmental pollution. MFI, Beta, CHA, aluminophosphate and silicoaluminophosphate molecular sieves and/or membranes have been prepared by the organic template-free secondary growth method,22–28 which provides the possibility of organic template-free synthesizing SAPO-34 molecular sieve membranes. In this present work, we have attempted to synthesize the continuous SAPO-34 molecular sieve membranes by the secondary growth method in the absence of organic templates. The obtained SAPO-34 membranes showed higher CO2–CH4 separation performance compared with the SAPO-34 membrane prepared with template.
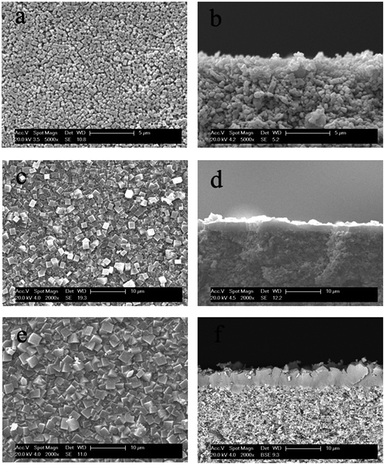
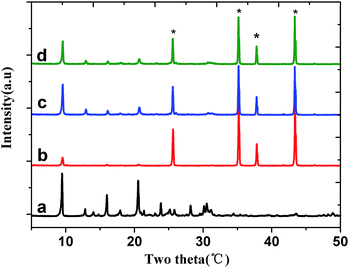
The morphology and crystal structure of the SAPO-34 molecular sieve membranes were characterized by SEM and XRD. As can be observed in Fig. 1(a) and b, the surface of the SAPO-34 seed layer is flat with a thickness of ca. 1.5 μm after spin coating. The SAPO-34 seeds were cubic with particle size of almost 0.6 μm. The XRD patterns of the seed crystals shown in Fig. 2(a) confirm the formation of the chabazite structure of SAPO-34 with characteristic reflections at 2θ = 9.5°, 20.55° and double peaks at 2θ = 25°, 31°.29 As shown in Fig. 1(c–f), after hydrothermal synthesis in a microwave oven at 403 K for 1 h and 453 K for 2 h, the continuous SAPO-34 molecular sieve membrane with a thickness of ca. 4 μm was successfully prepared on the surface of the support in the absence of organic templates. The SAPO-34 membrane is formed with well intergrown crystals. The XRD patterns of these membranes are shown in Fig. 2(b–d). Besides the alumina peaks marked by asterisks, the other peaks are attributed to typical CHA-type, indicating that the membrane synthesized are SAPO-34 molecular sieve membranes.
The SAPO-34 molecular sieve structure consists of two type of building units: double-six-ring (D6R) and chabazite cage. In the organic template-free secondary growth, K+ cation works as a structure-directing agent (SDA) in the formation of a precursor or cluster of silicate ions and water molecules. Furthermore, K+ cation replaces the TEA+ cation to balance the negative charge of SAPO-34 molecular sieve framework. This is in accordance with those reported in the previous paper.24,25
The SAPO-34 molecular sieves that were used for N2 adsorption–desorption isotherms experiments were collected from the bottom of the autoclave after membrane synthesis, respectively. The results, presented in Fig. 3, indicate that both molecular sieves are micropore materials. Fig. 4 shows the pore size distribution curves of the organic template-with SAPO-34 and organic template-free SAPO-34 molecular sieves calculated by DFT model. The distribution intensity of organic template-free SAPO-34 molecular sieves is high than that of organic template-with SAPO-34 molecular sieves, which shows that the organic template-free SAPO-34 molecular sieves has a high pore volume and a narrow pore size distribution compared with the organic template-with SAPO-34 molecular sieves.
 | ||
| Fig. 3 Nitrogen adsorption–desorption isotherms of organic template-with SAPO-34 and organic template-free SAPO-34 molecular sieves. | ||
 | ||
| Fig. 4 Pore size distributions curve of organic template-with SAPO-34 and organic template-free SAPO-34 molecular sieves. | ||
The quality of the molecular sieve membranes synthesized using organic templates could be detected by the light gas. A perfect molecular sieve membrane displayed gas tightness before the removal of organic templates. The SAPO-34 molecular sieve membrane with template was impervious to gas flow and the N2 permeance was below 10−11 mol m−2 s−1 Pa−1 at a temperature of 295 K, a pressure drop of 0.4 MPa. However, the organic template-free SAPO-34 molecular sieve membranes are not suitable by this method since the as-synthesized SAPO-34 membranes already possess open micropores.
The obtained SAPO-34 membranes were used to separate an equimolar CO2–CH4 mixture at 295 K for a pressure drop of 0.14 MPa and a permeate pressure of 0.1 MPa. As shown in Table 1, the organic template-free SAPO-34 membranes displayed CO2 permeance higher than 1.68 × 10−6 mol m−2 s−1 Pa−1. Furthermore, it exhibited CO2–CH4 separation selectivities as high as 256, which is 1.2 times that of the organic template-with SAPO-34 membrane. These values were stable for 64 h (Fig. 5). The excellent CO2–CH4 separation selectivities of the organic template-free SAPO-34 molecular sieve membranes might be attributed to the avoiding of defects in molecular sieve membranes led by organic template removal via high-temperature calcination.
| Membranes | Permeance (mol m−2 s−1 Pa−1) | CO2–CH4 selectivity | |
|---|---|---|---|
| CO2 × 106 | CH4 × 109 | ||
| Organic template-free SAPO-34-1 | 1.68 | 6.56 | 256 |
| Organic template-free SAPO-34-2 | 1.69 | 6.68 | 253 |
| Organic template-free SAPO-34-3 | 1.63 | 6.32 | 258 |
| SAPO-34-template | 1.72 | 8.07 | 213 |
 | ||
| Fig. 5 Permeances and selectivity of an equimolar CO2–CH4 mixture at 295 K, as a function of time, for organic template-free SAPO-34 membrane. | ||
Several other organic template-free SAPO-34 membranes were prepared by the secondary growth method under microwave-assisted heating. The CO2–CH4 separation performance results are listed in Table 1. The CO2–CH4 selectivity and the CO2 permeance for the SAPO-34 membranes were determined to be about 255 and 1.67 × 10−6 mol m−2 s−1 Pa−1, respectively and the values were reproducible.
In conclusion, the continuous SAPO-34 molecular sieve membranes have been prepared by the secondary growth in the absence of organic templates. The expense of organic templates, the calcination defects and the environment pollution could be avoided by the organic template-free synthesis. Moreover, the organic template-free synthesized SAPO-34 membranes displayed extremely high CO2–CH4 separation performance.
Experimental
SAPO-34 seeds and membranes were prepared using a hydrothermal treatment with microwave-heating. The molar ingredient of 1.0Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0P2O5
1.0P2O5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3SiO2
0.3SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2TEAOH
1.2TEAOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 52H2O was chosen for preparation of SAPO-34 seeds. In a typical synthesis, TEAOH (25%, Aldrich) and deionized water were mixed at room temperature and stirred at 500 rpm for 20 min. Subsequently, fumed silica (Aldrich) was added and the resulting solution was stirred for 2 h. Aluminum isopropoxide (98%, Aldrich) was then added and the resulting solution was stirred for 2 h. Phosphoric acid (85%, Aldrich) was added dropwise to the resultant solution to avoid the formation of dense gel particles. The solution was then transferred to a Teflon-PTFE autoclave. The crystallization was carried out in a microwave oven (MDS-6, Sineo Microwave Chemical Technology Co., Ltd.) at 453 K for 3 h. The precipitates were collected and washed by repeated centrifugation at 6000 rpm for 20 min and decantation until the pH value of the filtrate was ca. 7. The resulting precipitates were dried at 353 K overnight. Some of the seeds were kept for further analysis, the remaining seeds were calcined at 873 K for 6 h to remove the templates. The calcination heating rate was 1 K min−1.
52H2O was chosen for preparation of SAPO-34 seeds. In a typical synthesis, TEAOH (25%, Aldrich) and deionized water were mixed at room temperature and stirred at 500 rpm for 20 min. Subsequently, fumed silica (Aldrich) was added and the resulting solution was stirred for 2 h. Aluminum isopropoxide (98%, Aldrich) was then added and the resulting solution was stirred for 2 h. Phosphoric acid (85%, Aldrich) was added dropwise to the resultant solution to avoid the formation of dense gel particles. The solution was then transferred to a Teflon-PTFE autoclave. The crystallization was carried out in a microwave oven (MDS-6, Sineo Microwave Chemical Technology Co., Ltd.) at 453 K for 3 h. The precipitates were collected and washed by repeated centrifugation at 6000 rpm for 20 min and decantation until the pH value of the filtrate was ca. 7. The resulting precipitates were dried at 353 K overnight. Some of the seeds were kept for further analysis, the remaining seeds were calcined at 873 K for 6 h to remove the templates. The calcination heating rate was 1 K min−1.
The substrates used in the experiments were homemade α-Al2O3 disks with a thickness of 1.6 mm and a diameter of 20 mm, the average pore size of which was ca. 0.32 μm. The treatment procedure for the α-Al2O3 disks have been explained elsewhere.30 The treated α-Al2O3 substrate was seeded with SAPO-34 crystals (ca. 0.5 wt% in ethanol) by using a spin coater. During the spin-coating process, the substrates were vacuum-locked, and 0.5 mL drops of colloidal SAPO-34 suspension were introduced using a micro-injector. A uniform seed layer was prepared by spinning at a rate of 2000 rpm for 30 s, and drying at 333 K overnight. As a typical run, the SAPO-34 molecular sieve membrane was synthesized on the porous α-Al2O3 support by the organic template-free secondary growth method in the precursor suspension with a molar composition of 1.0Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 P2O5
1.0 P2O5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3SiO2
0.3SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.0K2O
3.0K2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 120H2O. The molar composition of the synthesis SAPO-34 membrane with template solution was 1.0 Al2O3
120H2O. The molar composition of the synthesis SAPO-34 membrane with template solution was 1.0 Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 P2O5
1.0 P2O5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3SiO2
0.3SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2TEAOH
1.2TEAOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 120H2O. The hydrothermal reaction was carried out in a microwave oven at 403 K for 1 h and 453 K for 2 h. Then, the autoclave was removed and cooled down to room temperature. The membranes prepared with template were calcined in air at 673 K for 4 h to remove the template. The calcination heating and cooling rates were 0.5 and 0.7 K min−1, respectively.
120H2O. The hydrothermal reaction was carried out in a microwave oven at 403 K for 1 h and 453 K for 2 h. Then, the autoclave was removed and cooled down to room temperature. The membranes prepared with template were calcined in air at 673 K for 4 h to remove the template. The calcination heating and cooling rates were 0.5 and 0.7 K min−1, respectively.
SEM images were recorded using a Philips XL30E scanning electron microscope (SEM). The X-ray diffraction (XRD) data was collected by a Rigaku D/max 2500v/pc diffractometer using Cu Kα radiation. The nitrogen adsorption–desorption experiments were carried on a Quantachrome Instruments apparatus. The detailed system of the separation experiments was described elsewhere.30 The CO2–CH4 (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) mixture was used in the permeation experiments performed at 295 K. The zeolite layer of the membrane faced the feed side. Controlled using a mass flowmeter, the CO2–CH4 streams were fed into the retentate side at a flow rate of 60 (STP) cm3 min−1, while the permeated gas on the permeate side was swept using helium at a flow rate of 5 (STP) cm3 min−1. A back-pressure regulator was used to control the total pressure and the pressure difference across the membrane. The pressure of the feed stream was kept at a certain press, while the permeate stream was kept at atmospheric pressure. After steady state was reached, the exit streams from the two sides were analyzed by an online gas chromatograph (Agilent 6890N) with a thermal-conductivity detector. The permeances of the CO2–CH4 gas mixture were investigated as a function of the total retentate pressure at 295 K. The flux was measured using a bubble flow meter.
50) mixture was used in the permeation experiments performed at 295 K. The zeolite layer of the membrane faced the feed side. Controlled using a mass flowmeter, the CO2–CH4 streams were fed into the retentate side at a flow rate of 60 (STP) cm3 min−1, while the permeated gas on the permeate side was swept using helium at a flow rate of 5 (STP) cm3 min−1. A back-pressure regulator was used to control the total pressure and the pressure difference across the membrane. The pressure of the feed stream was kept at a certain press, while the permeate stream was kept at atmospheric pressure. After steady state was reached, the exit streams from the two sides were analyzed by an online gas chromatograph (Agilent 6890N) with a thermal-conductivity detector. The permeances of the CO2–CH4 gas mixture were investigated as a function of the total retentate pressure at 295 K. The flux was measured using a bubble flow meter.
Notes and references
- L. Bastin, P. S. Brcia, E. J. Hurtado, J. A. C. Silva, A. E. Rodrigues and B. Chen, J. Phys. Chem. C, 2008, 112, 1575–1581 CAS.
- S. G. Li, J. G. Martinek, J. L. Falconer and R. D. Noble, Ind. Eng. Chem. Res., 2005, 44, 3220–3228 CrossRef CAS.
- R. W. Baker, Ind. Eng. Chem. Res., 2002, 41(6), 1393–1411 CrossRef CAS.
- W. J. Koros and R. Mahajan, J. Membr. Sci., 2000, 175(2), 181–196 CrossRef CAS.
- M. E. Davis, Nature, 2002, 417, 813–821 CrossRef CAS PubMed.
- Y. Hasegawa, T. Tanaka, K. Watanabe, B. H. Jeong, K. Kusakabe and S. Morooka, Korean J. Chem. Eng., 2002, 19, 309–313 CrossRef CAS.
- Q. G. Liu, T. H. Wang, H. C. Guo, C. H. Liang, S. L. Liu, Z. G. Zhang, Y. M. Cao, D. S. Su and J. S. Qiu, Microporous Mesoporous Mater., 2009, 120(3), 460–466 CrossRef CAS PubMed.
- X. Y. Yin, N. B. Chu, J. H. Yang, J. Q. Wang and Z. F. Li, Int. J. Greenhouse Gas Control, 2013, 15, 55–64 CrossRef CAS PubMed.
- J. van den Bergh, A. Tihaya and F. Kapteijin, Microporous Mesoporous Mater., 2010, 132(1–2), 137–147 CrossRef CAS PubMed.
- J. A. Bohrman and M. A. Carrion, Chem. Commun., 2012, 48, 5130–5132 RSC.
- H. B. T. Jeazet, C. Staudt and C. Janiak, Dalton Trans., 2012, 41, 14003–14027 RSC.
- M. U. M. Junaidi, C. P. Leo, A. L. Ahmad, S. N. M. Kamal and T. L. Chew, Fuel Process. Technol., 2014, 118, 125–132 CrossRef CAS PubMed.
- M. A. Carreon, S. G. Li, J. L. Falconer and R. D. Noble, Adv. Mater., 2008, 20(4), 729–732 CrossRef CAS PubMed.
- Y. Y. Tian, L. L. Fan, Z. Y. Wang, S. L. Qiu and G. S. Zhu, J. Mater. Chem., 2009, 19, 7698–7703 RSC.
- M. A. Carreon, S. G. Li, J. L. Falconer and R. D. Noble, J. Am. Chem. Soc., 2008, 130, 5412–5413 CrossRef CAS PubMed.
- R. F. Zhou, E. W. Ping, H. H. Funke, J. L. Falconer and R. D. Noble, J. Membr. Sci., 2013, 444, 384–393 CrossRef CAS PubMed.
- M. C. Lovallo, A. Gouzinis and M. Tsapatsis, AIChE J., 1998, 44(8), 1903–1913 CrossRef CAS PubMed.
- S. G. Li, V. A. Tuan, J. L. Falconer and R. D. Noble, Microporous Mesoporous Mater., 2003, 58(2), 137–154 CrossRef CAS.
- A. Tavolaro, A. Julbe, C. Guizard, A. Basile, L. Cot and E. Drioli, J. Mater. Chem., 2000, 10, 1131–1137 RSC.
- D. Coutinho and K. J. Balkus Jr, Microporous Mesoporous Mater., 2002, 52(2), 79–91 CrossRef CAS.
- J. Caro and M. Noack, Microporous Mesoporous Mater., 2008, 115, 215–233 CrossRef CAS PubMed.
- W. H. Yuan, Y. S. Lin and W. S. Yang, J. Am. Chem. Soc., 2004, 126, 472–478 CrossRef PubMed.
- Y. T. Tang, X. F. Liu, S. F. Nai and B. Q. Zhang, Chem. Commun., 2014, 50, 8834–8837 RSC.
- Y. Hasegawa, H. Hotta, K. Sato, T. Nagase and F. Mizukami, J. Membr. Sci., 2010, 347, 193–196 CrossRef CAS PubMed.
- X. Li, H. Kita, H. Zhu, Z. Zhang, K. Tanaka and K. Okamota, Microporous Mesoporous Mater., 2011, 143, 270–276 CrossRef CAS PubMed.
- K. Kunii, K. Narahara and S. Yamanaka, Microporous Mesoporous Mater., 2002, 52, 159–167 CrossRef CAS.
- H. Nakayama, H. Kataoka, Y. Taketani, C. Sumita and M. Tsuhako, Microporous Mesoporous Mater., 2002, 51(1), 7–15 CrossRef CAS.
- Y. Li, G. S. Dou, G. F. Ma, Q. K. Du, Y. X. Shen and Y. Li, CN 102134081, 2011.
- B. M. Lok, C. A. Messina, R. L. Patton, R. T. Gajek, T. R. Cannan and E. M. Flanigen, J. Am. Chem. Soc., 1984, 106, 6092–6093 CrossRef CAS.
- L. Lang, X. F. Liu and B. Q. Zhang, Appl. Surf. Sci., 2008, 254, 2353–2358 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |