A highly efficient and magnetically retrievable functionalized nano-adsorbent for ultrasonication assisted rapid and selective extraction of Pd2+ ions from water samples†
Abstract
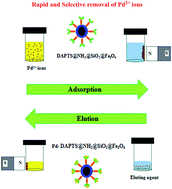
A novel magnetically retrievable core–shell structured nano-adsorbent (DAPTS@NH2@SiO2@Fe3O4) was fabricated for selective extraction of Pd2+ ions from water samples. The nano-adsorbent was well characterized by XRD, TEM, FE-SEM, FTIR, EDS, ED-XRF and VSM. The influence of various factors on adsorption and desorption such as the amount of nano-adsorbent, pH variation, adsorption time, eluting agent type and its volume were investigated. The maximum adsorption capacity was found to be 64.8 (mg g−1) which perfectly fitted with a Langmuir isotherm model of adsorption. Also, the adsorption time of 5 minutes suggested faster adsorption kinetics which was in agreement with a pseudo second order kinetic model. Besides, the presence of a rich magnetic core facilitated the ease of separation of the nanoadsorbent. The overall results suggested the potential use of the nano-adsorbent for the faster, selective and quantitative extraction of Pd2+ ions from aqueous and catalytic converter samples.

 Please wait while we load your content...
Please wait while we load your content...