Using nano-QSAR to determine the most responsible factor(s) in gold nanoparticle exocytosis†
Abstract
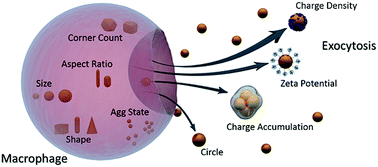
There are, to date, few general answers to fundamental questions related to the interactions of nanoparticles (NPs) with living cells. Studies reported in the literature have delivered only limited principles about the nano–bio interface and thus the biological behavior of NPs is yet far from being completely understood. Combining computational tools with experimental approaches in this regard helps to precisely probe the nano–bio interface and allows the development of predictive and descriptive relationships between the structure and the activity of nanomaterials. In the present contribution, a nano-quantitative structure-activity relationship (nano-QSAR) model has been statistically established using the Partial Least Squares Regression (PLSR) model. Also, variable importance on PLS projections (VIP) has been used to find the most responsible factors in NP exocytosis. Physicochemical properties of a set of different sized gold NPs with different surface coatings were strongly correlated to their exocytosis in macrophages. The results suggest that among the pool of physicochemical properties defined as nano-descriptors, charge density and surface charge seem to be the paramount factors leading to higher exocytosis values. Furthermore, charge accumulation and circularity of NPs are in the next level of priority among other nano-descriptors. The regression based nano-QSAR model reported here is satisfactory in both statistical quality and interpretability. The results could serve as a quantitative framework for better understanding the mechanisms that govern the interactions at the nano–bio interface.

 Please wait while we load your content...
Please wait while we load your content...