Simultaneous ultrasonic-assisted removal of malachite green and safranin O by copper nanowires loaded on activated carbon: central composite design optimization
Abstract
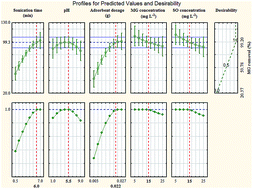
The present study investigates the simultaneous ultrasound-assisted adsorption of malachite green (MG) and safranin O (SO) dyes from aqueous solutions by ultrasound-assisted adsorption onto copper nanowires loaded on activated carbon (Cu-NWs-AC). In this study a novel and green approach is described for the synthesis of Cu nanowires. This novel material was characterized using different techniques such as FESEM, XRD, EDS, and UV-vis. The effects of variables such as sonication time, pH, adsorbent dosage, and initial dye concentrations on simultaneous dye removals were studied and optimized by a central composite design (CCD) combined with a desirability function (DF). A good agreement between experimental and predicted data using an optimal model in this study was observed. These results indicated that when a small amount of proposed adsorbent (0.022 g) was applied, it resulted in simultaneous removal of 15 mg L−1 of malachite green and 15 mg L−1 of safranin O (>99%) in a short time (6.0 min) at a pH of 5.5.

 Please wait while we load your content...
Please wait while we load your content...