Fabrication of carbon black coated flexible polyurethane foam for significantly improved fire safety†
Wei Wangab,
Haifeng Panab,
Bin Yuab,
Ying Pana,
Lei Song*a,
Kim Meow Liewbc and
Yuan Hu*ab
aState Key Laboratory of Fire Science, University of Science and Technology of China, 96 Jinzhai Road, Hefei, Anhui 230026, People's Republic of China. E-mail: yuanhu@ustc.edu.cn; leisong@ustc.edu.cn; Fax: +86-551-63601664; Tel: +86-551-63601664
bSuzhou Key Laboratory of Urban Public Safety, Suzhou Institute of University of Science and Technology of China, 166 Ren'ai Road, Suzhou, Jiangsu 215123, People's Republic of China
cDepartment of Architecture and Civil Engineering, City University of Hong Kong, Tat Chee Avenue Kowloon, Hong Kong, People's Republic of China
First published on 9th June 2015
Abstract
Fire resistant coatings, composed of nanosized carbon black (CB) and polyurethane acrylate (PUA), were synthesized through a facile and low-cost method to improve the fire safety and thermal stability of flexible polyurethane foam (FPU). Scanning electron microscopy and total reflection Fourier transform infrared analysis results demonstrated the successful deposition of the coating on the surface of FPU foam and the morphology change of FPU with different concentrations of CB. Thermogravimetric analysis results revealed that the thermal stability of the coated FPU foams was significantly improved. The char residue of FPU/CB 8% was increased to 28.4% from 4.0 wt% of pure FPU. The coated FPU foam with a CB concentration of 8 wt% exhibited a great reduction of 80% in peak heat release rate (pHRR), attributing to the physical barrier effect of the CB filled coating. The observation of the char residue indicated that FPU/CB 4% exhibited the best shape char residue without collapse and cracking. Simultaneously, the SEM images of the char residues of the coated FPU foams showed that carbon black based coating promoted the generation of a compact char layer, suggesting a good physical blocking effect. Raman spectroscopy also confirmed that the graphitization degree of char residue FPU/CB 4% was the lowest, with the more stable structure. The coated FPU foam also has a certain inhibitory effect on the smoke release.
1 Introduction
Flexible polyurethane foams (FPUF) are widely used in various fields of decoration, furniture and carpet underlay of automobiles1,2 due to its excellent cushioning and physical properties.3–5 With the rapid development of the automobile manufacturing industry, the demand of FPUF has greatly increased.6 However, FPUF, as highly cellular polymers, are easy to ignite, burn rapidly with a high heat release rate, release smoke and do not have any residual char.7–9 Due to increasingly strict standards being developed in traffic safety regulations, increasing attention has been focused on improving the flame-retardant properties of FPUF.10,11Generally, flame retardant additives based on halogens, phosphorus, nitrogen, boron, silicon can improve the flame retardancy of FPUFs on the basis of condensed-phase and/or gas-phase mechanisms.8,12 Nevertheless, some flame retardants release toxic gases along with smoke during combustion, especially the halogenated compounds.13 To solve these issues, researchers have explored various methods.10 In recent years, various nano-additives have been employed as flame retardants, such as polyhedral oligomeric silsesquioxane (POSS),14 carbon nanotubes (CNTs),15 graphene,16 and molybdenum disulfide,17 to endow polymers with flame retardancy. Compared with halogen containing flame retardants, these sheets or tubular flame retardants exhibit a physical barrier effect during burning, which reduces the spread and production of smoke.18 Recently, the layer by layer (LbL) technique has been applied to prepare efficient flame retardants,19,20 improve the flame retarding performance, and reduce the smoke toxicity of FPUF.21 Pan deposited montmorillonite (MMT) and multiwall carbon nanotubes (CNT) on the surface of flexible PU foam by the LbL technique, which exhibited a remarkable reduction in peak heat release rate (PHRR), but did not significantly reduce total heat release (THR), indicating that the MMT/CNT based coating acts as a physical insulating barrier to delay the heat release.21 Although the LbL technology and nano-additives (CNTs) improve the flame retardancy of FPUF, the high cost makes it difficult to promote their use in practical applications. Therefore, exploring a relatively low cost technology, which is able to effectively improve the flame retardant properties of FPUF, is imperative.
Owing to its abundant source, low cost, low density and excellent heat stability,22,23 nanoscale carbon black (CB) is widely used in electrochemistry, mechanical enhancements, flame retardant polymers and other fields.24–30 In prior studies, flame retardant polymers incorporating different loadings of carbon black have been reported: Wen et al. synthesized polypropylene (PP)/carbon black nanocomposites by melt compounding and investigated the effect of nanofiller loadings on the thermal and flammability properties of PP.31,32 The obtained nanocomposites displayed dramatically enhanced thermal stability, both under nitrogen and air, and improved flame retardancy to some extent. Dittrich et al. investigated the effect of different carbon materials, including carbon black, multiwall nanotubes, expanded graphite, multi-layer graphene and graphene, on the fire resistance of PP,33,34 as carbon black can effectively decrease the HRR value. Carbon black is usually used as a nano-additive in polymer matrices, but the application in improving the flame retardant properties of PUF as a coating on the surface of the matrix is rarely reported.
Herein, we prepared a coating that consisted of nanosized CB and polyurethane acrylate (PUA) with ethanol as a solvent. Nanoscale CB was fixed on the surface of FPUF by a PUA cross-linked network using thermal crosslinking technology. To observe the surface topography of FPUF after the crosslinking reaction, scanning electron microscopy (SEM) was employed. The combustion performance and the thermal properties of FPUF/CB were evaluated by a cone calorimeter; thermal degradation behavior was investigated using thermogravimetric analysis (TGA). The method used here is very easy to operate and the materials are cost-effective and environmentally friendly. These advantages provide a feasible strategy for the realization of industrialized production.
2 Experimental
2.1 Materials
Flexible polyurethane foam (DW30) was purchased from Jiangsu Lvyuan New Material Co., Ltd. Polyurethane acrylate (PUA) was received from Guangzhou Tongyi New Material Co., Ltd. Ethanol was obtained from Sinopharm Chemical Reagent Co. Ltd. Nanosized carbon black (CB) was purchased from Qingdao Tianhe Graphite Co. Ltd., China. 2,2-Azobisisobutyronitrile (AIBN) was purchased from Sinopharm Chemical Reagent Co. Ltd.2.2 Preparation of CB/PUA coating
The preparation of the coating with different CB contents: 1, 2, 4 and 8 wt%, is described as follows. The weight fraction of CB means the weight proportion of carbon black in the whole suspension. The formula of the suspension is provided in Table 1. For the coating with 1 wt% loading, 6 g CB, 20 g PUA and 0.1 g AIBN were dispersed in 574 g of ethanol in a 1000 mL beaker with constant stirring and ultrasonic agitation overnight. The coatings with four different CB loadings were prepared using the same method.| Num | Carbon black/g | PUA/g | Ethyl alcohol/g | AIBN/g | Weight gain/% |
|---|---|---|---|---|---|
| FPU/CB 1% | 6 | 20 | 574 | 0.1 | 7.2 |
| FPU/CB 2% | 12 | 20 | 568 | 0.1 | 11.6 |
| FPU/CB 4% | 24 | 20 | 556 | 0.1 | 24.6 |
| FPU/CB 8% | 48 | 20 | 532 | 0.1 | 37.5 |
2.3 Preparation of FPU/CB/PUA coating (FPU/CB)
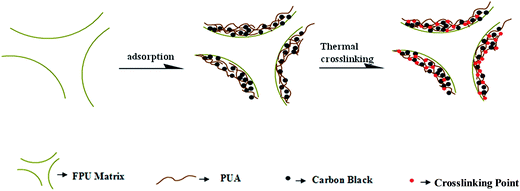
Prior to deposition, the FPU foam was pre-soaked in ethanol for 1 min to create a compatible surface. Then, the FPU foam was soaked in the CB/PUA coating solution for 5 min to improve the adhesion to the substrates. Subsequently, the FPU foam was wringed to expel the excess coating in the FPU foam. After the completion of the deposition of the desired coating, FPU foam was dried at 60 °C for 1 h to evaporate the ethanol. Finally, the thermal crosslinking reaction of PUA was conducted at 180 °C for 16 hours. The process of adsorption and thermal crosslinking is shown in Scheme 1. Four different loadings of coatings were prepared according to the same process.2.4 Characterization
The morphologies of control and coated FPU foams were observed using scanning electron microscopy (SEM, AMRAY1000B, Beijing R&D Center of the Chinese Academy of sciences, China). The samples were coated with a gold layer before observation.The combustion test was performed on a cone calorimeter (Fire Testing Technology, UK) according to ISO 5660 standard procedures with specimens of 100 × 100 × 25 mm3. Each specimen was exposed horizontally to 35 kW m−2 external heat flux.
FTIR spectra were recorded on a Nicolet MAGNA-IR 750 FTIR spectrometer. Potassium bromide (KBr) was ground in a mortar with a pestle and enough solid samples were ground with KBr to make a 1 wt% mixture for the KBr pellets. The mixture of KBr and the samples was pressed into a tablet for characterization, which was then placed in a ventilated oven. The transition mode was used, and the wavelength range was set from 4000 to 500 cm−1.
Attenuated total reflection Fourier transform infrared (ATR-FTIR) spectra, in the frequency wavenumber region of 4000–400 cm−1 at a 4 cm−1 resolution, were recorded by a Nicolet 6700 spectrometer (Thermo-Nicolet) using 32 scans.
Thermogravimetric analysis (TGA) of samples was carried out with a TGA-Q5000 apparatus (TACompany, USA) from 50 to 600 °C at a heating rate of 20 °C min−1. The weight of all the samples was kept within 3–5 mg in an open platinum pan.
Laser Raman spectroscopy measurements were carried out at room temperature with a SPEX-1403 laser Raman spectrometer (SPEX Co., USA).
3 Results and discussion
3.1 Characterization of the pure FPU foam and coated FPU foams
ATR-FTIR spectroscopy was used to measure the structural change of the surface of FPU samples. As shown in Fig. 1, three absorption peaks at 1098, 1222 and 1540 cm−1 are attributed to the non-symmetric stretching vibration of C–O–C, the stretching vibration of aromatic C–O, deformation and stretching vibrations of N–H in FPU foam, respectively.35,36 The absolute intensity of these three bands is decreased with the increase of the coating mass, which is ascribed to the overlaying of CB based coating.SEM was used to describe the surface morphology of the samples after the thermal crosslinking reaction. The SEM images of pure and coated FPU foams are shown in Fig. 2. The surface of the pure FPU foam skeleton is smooth and clean. However, these coated FPU foams exhibit much rougher and thicker surface than that of neat PUA, owing to the coverage of the PUA/CB coating after thermal crosslinking reaction. From Fig. 2b–d, it can be easily seen that the surface of the FPU foam skeleton becomes increasingly rough with increasing concentration of CB. When the CB concentration is low, the PUA crosslinking network can effectively cover and pack the CB particles, making the surface of FPU relatively smooth. When the CB concentration reaches 4%, the PUA crosslinking network cannot easily cover the CB particles, making the surface rough.
3.2 Thermal properties
Thermogravimetric analysis (TGA) and derivative thermogravimetric (DTG) curves provide direct information about the thermal stability and degradation mechanism by measuring the weight loss of samples. Fig. 3 illustrates the TGA and DTG curves of pure FPU and the coated FPU foams under nitrogen atmosphere, and the related data are shown in Table 2. The temperature corresponding to 5 wt% weight loss (T−5%) is defined as the initial degradation temperature. The mid-point temperature of the degradation (T−50%) and the solid residue left at 600 °C are also obtained from TGA curves. It is noted that pure FPU and coated FPU foams show a two-stage thermal degradation process. The first region from 270 to 300 °C corresponds to the release of physically absorbed water and the liberation of toluene diisocyanate (TDI), which is attributed to the depolymerization of the urethane and the bi-substitution of urea groups.37 Compared with the pure FPU foam, the other coated FPU foams show a higher initial degradation temperature, which is mostly due to the high thermal stability and blocking effect of nanosized carbon black based coating. The second region from 350 to 400 °C is the main pyrolysis region, which is attributed to the dehydration and decarboxylation reactions, producing combustible gases like aldehydes, ketones, ethers. For the DTG curves in the second region, we obtained the information that the temperature (Tmax2) of the FPU foam composites was much higher than pure FPU foam, indicating that the adsorption of the nanosized CB effectively prevents the permeation of the heat from the surface, and thus reduces the mass loss rate (MLR) and improves the thermal stability of FPU foam.| Num | T−5% (°C) | T−50% (°C) | Tmax2 (°C) | Char residue at 600 °C (%) |
|---|---|---|---|---|
| Pure FPU | 246 ± 8 | 354 ± 1 | 358 ± 6 | 4.2 ± 0.2 |
| FPU/CB 1% | 258 ± 9 | 373 ± 1 | 378 ± 3 | 8.0 ± 0.7 |
| FPU/CB 2% | 255 ± 11 | 371 ± 7 | 384 ± 12 | 13.7 ± 1.5 |
| FPU/CB 4% | 260 ± 6 | 388 ± 6 | 380 ± 9 | 21.4 ± 2.1 |
| FPU/CB 8% | 258 ± 10 | 383 ± 9 | 387 ± 11 | 26.8 ± 1.6 |
3.3 Flammability properties
Cone calorimeter was employed to evaluate the flame retardancy of pure FPU foam and FPU foam coated with CB/PUA. Fig. 4 and 5 present the heat release rate (HRR) and total heat release (THR) curves of the control and CB coated FPU foams. The specific data obtained from cone calorimeter tests are listed in Table 3. Pure FPU foam shows two HRR peaks, corresponding to the pyrolysis of isocyanate and polyol, respectively. Adhering CB filled coating onto the surface of the FPU foam matrix significantly reduces the peak heat release rate (pHRR). Compared with the control sample (712 kW m−2), the pHRR value of FPU/CB 8% (138 kW m−2) shows an 80% reduction in pHRR, the optimal fire safety among all the samples. CB nanoparticles at elevated temperatures could form a gelled-ball crosslink network,31,32 which acts as a physical insulating barrier to reduce the release of evolved gases. However, the CB filled coating has little influence on the reduction in THR, suggesting that the CB based coating acts as a physical insulating barrier to delay the HRR, but it does not significantly reduce the final THR. It can be seen that the THR values of FPU/CB 4% and FPU/CB 8% are slightly higher than those of other samples; this is probably because as the weight gain increases, the contents of PUA also increase, and the combustion of PUA leads to the increase of THR values. In addition, the flammability reduction is directly aligned with the coating concentration. Gradual reduction in pHRR is achieved with over-increasing the concentration of CB. In particular, the coated FPU foams prepared from high CB concentrations (4 wt% and 8 wt%) are able to completely eliminate the second peak and largely extend the time for complete combustion.| Num | Peak HRR (kW m−2) | THR (kJ g−1) | Peak SPR (m2 s−1) |
|---|---|---|---|
| Pure FPU | 748 ± 36 | 17.4 ± 0.7 | 0.355 ± 0.012 |
| FPU/CB 1% | 650 ± 28 | 16.2 ± 0.8 | 0.335 ± 0.009 |
| FPU/CB 2% | 457 ± 31 | 16.4 ± 1.5 | 0.336 ± 0.013 |
| FPU/CB 4% | 272 ± 25 | 17.8 ± 1.1 | 0.344 ± 0.019 |
| FPU/CB 8% | 164 ± 19 | 18.1 ± 1.1 | 0.328 ± 0.014 |
3.4 The residue analysis of the coated FPU foams
To better understand the burning behavior of the coated FPU foams, the residues of the coated FPU foams after cone calorimeter tests were collected, as shown in Fig. 6. Generally, effective residue, i.e., fast formation, denseness, thickness, and/or formation of little cracks, contributes to improving the flame retardancy of polymeric materials.38 As can be seen from Fig. 6a, there is limited amount of residue found in the control FPU foam. For the samples of FPU/CB 1% and FPU/CB 2%, there are some residues after combustion, because the amount of nanosized CB is not enough to effectively protect the structure of FPU foam. As the CB loading increases to 4 wt%, it can be seen that the residue exhibits smooth appearance; the shape of the residue is consistent with the sample before burning. Moreover, the residue is gradually increased, suggesting the gradual improvement in thermal stability. This result is explained by the fact that the CB based layer effectively protects the structure of FPU foam. | ||
| Fig. 6 Digital photographs of char residue for the control FPU (a), FPU/CB 1% (b), FPU/CB 2% (c) and FPU/CB 4% (d). | ||
To observe the surface morphology and identify the mechanism of the PUA/CB crosslinking coating, the SEM images of the residue were obtained, and are displayed in Fig. 7. As can be seen from Fig. 7a and b (FPU/CB 1% under different magnification), the residue collapse and break obviously, and cannot maintain the original shape of FPU. This is because a low concentration of CB is unable to completely cover the surface of the FPU skeleton. When the combustion occurs, heat and oxygen are easily transferred. Images c and d for FPU/CB 4% with different magnification indicate the FPU skeleton is maintained, and has no collapse and breakage. Compared with the SEM images of FPU at the same concentration before combustion, the morphology almost have no changes, and the surface is still intact and wrapped by CB layer. This char layer plays the role of a physical barrier and maintains the shape of FPU during combustion.
Raman spectroscopy is often used to explore the chemical structure of residues. Herein, it is a helpful measurement to characterize the graphitic structure of the residue. Fig. 8 shows the Raman spectra of the residue of coated FPU foams after cone calorimeter tests. From the spectra, it can be seen that all the samples exhibit two visible bands, namely, the D-band (1350 cm−1), associated with the vibration of carbon atoms with dangling bonds in the plane terminations of disordered graphite or glass carbons, and the G-band (1580 cm−1), corresponding to the vibration of sp2-bonds carbon atoms in graphite layers.39 Generally, the ratio of the intensity of the D and G bands (ID/IG) is used to evaluate the graphitization degree of residue: the lower the value of ID/IG, the higher is the thermal stability of char residue. Raman results show that the ratio of ID/IG follows the order: pure FPU > FPU/CB 1% > FPU/CB 2% > FPU/CB 4%. This result is associated with the images of the residue presented above.
 | ||
| Fig. 8 Raman spectra of residual char of pure FPU (a), FPU/CB 1% (b), FPU/CB 2% (c) and FPU/CB 4% (d) after cone test. | ||
3.5 Fire test of the control and coated FPU foams in open space
The combustion photos of the control and coated FPU foams at a time of 50 s and the final char residues under air are presented in Fig. 9. The combustion process of the control and coated FPU foams were recorded in a digital video and the related videos are provided in ESI† (a Sony HDR-CX360E was used to capture the combustion video). Obviously, after the deposition of the coating formed by CB and PUA, there is no dripping during the combustion process and no burning of cotton wool. Simultaneously, by increase in the concentration of CB, the rate of spread of the fire became slower; this property can be crucial for the escape time of people when the fire accident occurs. Through the observation of the video times, we can find that combustion time became longer as the concentration of CB is increased. This is because CB can improve the thermal stability and the compactness of the coating, which can effectively delay the permeation of heat and postpone the flammable volatile gases from escaping to the surface of the FPU foam. Therefore, volatile gases are liberated at a low release rate, which decreases the heat release rate (HRR). As shown in the image of char residues, pure FPU foam was easily ignited, rapidly spread, dripped and left nothing; and the char residue of coated FPU foams increased evidently after the deposition of coating. | ||
| Fig. 9 The combustion photos of the control and coated FPU foams at the time of 50 s and the final char residues under air. | ||
3.6 Flame retardant mechanism
It is well known that carbon black has excellent thermal stability. Therefore, we utilized polyurethane acrylate to adhere CB on the surface of FPU foam, and polyurethane acrylate acted as an important role to promote the formation of a compact layer after thermal crosslinking treatment, which could fix CB particles on the surface of FPU foam. As has been previously reported, CB nanoparticles at elevated temperatures can form a gelled-ball crosslink network, which acts as a barrier and plays a major role in the improvement of fire resistance.31,32 In addition, the layer-by-layer technique is also used to improve the flame retardancy of FPU by depositing nanofillers on its surface, and the mechanism is mainly ascribed to the physical barrier of the coating.21,40,41 Therefore, the probable flame retardancy was proposed as follows: polyurethane acrylate promotes the formation of a compact CB based layer after thermal crosslinking treatment, which could act as a protective layer. As FPU foam was exposed to heat radiation, the compact layer can delay the permeation of heat. Simultaneously, this protective layer can also have the barrier effect on the permeation of oxygen and flammable volatile gases, which can slow down the combustion reaction and decrease the peak heat release rate (pHRR). This has been demonstrated in the cone calorimeter test, which exhibited a great reduction in pHRR. Moreover, we can also verify this point through the changes in the video time. Due to the slow release of volatile gases, the combustion process lasts longer; the cone calorimeter test also explains this phenomenon. The possible flame retardant mechanism has been depicted in Fig. 10.4 Conclusion
The coating containing nanosized carbon black and polyurethane acrylate was deposited on the surface of flexible polyurethane (FPU) foam by thermal crosslinking technology to enhance its thermal stability and combustion properties. SEM images and ATR-FTIR spectra of the pure and coated FPU foams showed the significant change of surface structure after the deposition and thermal crosslinking by the coating. TGA and DTG analysis results revealed that the thermal stability of the coated FPU foams was remarkably improved. The increase in char residue was easily observed. The coated FPU foams with the CB concentration of 8 wt% had a great reduction in peak HRR, 80%, which is attributed to the physical barrier blocking effect of the coating. Raman spectroscopy confirmed that the graphitization degree of char residue FPU/CB 4% was the lowest because of the more stable structure. Macro fire testing can also illustrate the improvement of the FPU combustion performance concisely and intuitively.Acknowledgements
The work was financially supported by the National Basic Research Program of China (973 Program) (2012CB719701), National Key Technology R&D Program (2013BAJ01B05), the National Natural Science Foundation of China (51473154), and National Natural Science Foundation of China (51276054).References
- J. Lefebvre, B. Bastin, M. Le Bras, S. Duquesne, R. Paleja and R. Delobel, Polym. Degrad. Stab., 2005, 88, 28–34 CrossRef CAS PubMed.
- A. Konig, U. Fehrenbacher, T. Hirth and E. Kroke, J. Cell. Plast., 2008, 44, 469–480 CrossRef PubMed.
- S. Liang, M. Neisius, H. Mispreuve, R. Naescher and S. Gaan, Polym. Degrad. Stab., 2012, 97, 2428–2440 CrossRef CAS PubMed.
- J. Lefebvre, B. Bastin, M. Le Bras, S. Duquesne, C. Ritter, R. Paleja and F. Poutch, Polym. Test., 2004, 23, 281–290 CrossRef CAS PubMed.
- C. Chao and J. Wang, Combust. Flame, 2001, 127, 2252–2264 CrossRef CAS.
- H. Singh and A. Jain, J. Appl. Polym. Sci., 2009, 111, 1115–1143 CrossRef CAS PubMed.
- A. Gharehbaghi, R. Bashirzadeh and Z. Ahmadi, J. Cell. Plast., 2011 DOI:0021955X11414789.
- E. D. Weil and S. V. Levchik, J. Fire Sci., 2004, 22, 183–210 CrossRef CAS PubMed.
- A. König and E. Kroke, Polym. Adv. Technol., 2011, 22, 5–13 CrossRef PubMed.
- C. Q. Wang, F. Y. Ge, J. Sun and Z. S. Cai, J. Appl. Polym. Sci., 2013, 130, 916–926 CrossRef CAS PubMed.
- C. Denecker, J. Liggat and C. Snape, J. Appl. Polym. Sci., 2006, 100, 3024–3033 CrossRef CAS PubMed.
- M. Modesti, A. Lorenzetti, S. Besco, D. Hrelja, S. Semenzato, R. Bertani and R. Michelin, Polym. Degrad. Stab., 2008, 93, 2166–2171 CrossRef CAS PubMed.
- A. Andersson, A. Magnusson, S. Troedsson, S. Lundmark and F. H. Maurer, J. Appl. Polym. Sci., 2008, 109, 2269–2274 CrossRef CAS PubMed.
- A. Dasari, Z.-Z. Yu, Y.-W. Mai, G. Cai and H. Song, Polymer, 2009, 50, 1577–1587 CrossRef CAS PubMed.
- G. Huang, S. Wang, P. a. Song, C. Wu, S. Chen and X. Wang, Composites, Part A, 2014, 59, 18–25 CrossRef CAS PubMed.
- X. Wang, L. Song, H. Yang, H. Lu and Y. Hu, Ind. Eng. Chem. Res., 2011, 50, 5376–5383 CrossRef CAS.
- Z. Matusinovic, R. Shukla, E. Manias, C. G. Hogshead and C. A. Wilkie, Polym. Degrad. Stab., 2012, 97, 2481–2486 CrossRef CAS PubMed.
- B. Wang, K. Zhou, B. Wang, Z. Gui and Y. Hu, Ind. Eng. Chem. Res., 2014, 53, 12355–12362 CrossRef CAS.
- F. Carosio, A. Di Blasio, J. Alongi and G. Malucelli, Polymer, 2013, 54, 5148–5153 CrossRef CAS PubMed.
- F. Carosio, F. Cuttica, A. Di Blasio, J. Alongi and G. Malucelli, Polym. Degrad. Stab., 2014, 32, 16674–16680 Search PubMed.
- H. Pan, Y. Pan, W. Wang, L. Song, Y. Hu and K. M. Liew, Ind. Eng. Chem. Res., 2014, 53, 14315–14321 CrossRef CAS.
- C.-K. Leong and D. Chung, Carbon, 2004, 42, 2323–2327 CrossRef CAS PubMed.
- F. Delor-Jestin, J. Lacoste, N. Barrois-Oudin, C. Cardinet and J. Lemaire, Polym. Degrad. Stab., 2000, 67, 469–477 CrossRef CAS.
- S. Praveen, P. Chattopadhyay, P. Albert, V. Dalvi, B. Chakraborty and S. Chattopadhyay, Composites, Part A, 2009, 40, 309–316 CrossRef PubMed.
- C. Portet, G. Yushin and Y. Gogotsi, Carbon, 2007, 45, 2511–2518 CrossRef CAS PubMed.
- J. Huang, B. G. Sumpter, V. Meunier, G. Yushin, C. Portet and Y. Gogotsi, J. Mater. Res., 2010, 25, 1525–1531 CrossRef CAS.
- M. Sumita, K. Sakata, S. Asai, K. Miyasaka and H. Nakagawa, Polym. Bull., 1991, 25, 265–271 CrossRef CAS.
- F. Gubbels, S. Blacher, E. Vanlathem, R. Jérôme, R. Deltour, F. Brouers and P. Teyssie, Macromolecules, 1995, 28, 1559–1566 CrossRef CAS.
- X. Wen, J. Gong, H. Yu, Z. Liu, D. Wan, J. Liu, Z. Jiang and T. Tang, J. Mater. Chem., 2012, 22, 19974–19980 RSC.
- F. Gubbels, R. Jérôme, P. Teyssie, E. Vanlathem, R. Deltour, A. Calderone, V. Parente and J.-L. Brédas, Macromolecules, 1994, 27, 1972–1974 CrossRef CAS.
- X. Wen, N. Tian, J. Gong, Q. Chen, Y. Qi, Z. Liu, J. Liu, Z. Jiang, X. Chen and T. Tang, Polym. Adv. Technol., 2013, 24, 971–977 CrossRef CAS PubMed.
- X. Wen, Y. Wang, J. Gong, J. Liu, N. Tian, Y. Wang, Z. Jiang, J. Qiu and T. Tang, Polym. Degrad. Stab., 2012, 97, 793–801 CrossRef CAS PubMed.
- B. Dittrich, K.-A. Wartig, D. Hofmann, R. Mülhaupt and B. Schartel, Polym. Degrad. Stab., 2013, 98, 1495–1505 CrossRef CAS PubMed.
- B. Dittrich, K. A. Wartig, D. Hofmann, R. Mülhaupt and B. Schartel, Polym. Adv. Technol., 2013, 24, 916–926 CrossRef CAS PubMed.
- X. Wang, Y. Hu, L. Song, H. Yang, W. Xing and H. Lu, J. Mater. Chem., 2011, 21, 4222–4227 RSC.
- Y. Liu, J. Ma, T. Wu, X. Wang, G. Huang, Y. Liu, H. Qiu, Y. Li, W. Wang and J. Gao, ACS Appl. Mater. Interfaces, 2013, 5, 10018–10026 CAS.
- M. Zammarano, R. H. Krämer, R. Harris, T. J. Ohlemiller, J. R. Shields, S. S. Rahatekar, S. Lacerda and J. W. Gilman, Polym. Adv. Technol., 2008, 19, 588–595 CrossRef CAS PubMed.
- R. Krämer, M. Zammarano, G. Linteris, U. Gedde and J. Gilman, Polym. Degrad. Stab., 2010, 95, 1115–1122 CrossRef PubMed.
- X. Wang, Y.-T. Pan, J.-T. Wan and D.-Y. Wang, RSC Adv., 2014, 4, 46164–46169 RSC.
- F. Carosio, G. Fontaine and J. Alongi, ACS Appl. Mater. Interfaces, 2015, 22, 12158–12167 Search PubMed.
- H. Pan, W. Wang and Y. Pan, ACS Appl. Mater. Interfaces, 2015, 1, 101–111 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra06170a |
| This journal is © The Royal Society of Chemistry 2015 |