Nanoarrays: design, preparation and supercapacitor applications
Di Guoa,
Linfei Lai*b,
Anmin Cao*c,
Huakun Liud,
Shixue Doud and
Jianmin Ma*a
aKey Laboratory for Micro-Nano Optoelectronic Devices of Ministry of Education, State Key Laboratory for Chemo/Biosensing and Chemometrics, Hunan University, Changsha, P. R. China. E-mail: nanoelechem@hnu.edu.cn
bDivision of Physics and Applied Physics, School of Physical and Mathematical Sciences, Nanyang Technological University, Singapore, 637371, Singapore. E-mail: LaiLF@ntu.edu.sg
cKey Laboratory of Molecular Nanostructure and Nanotechnology, Institute of Chemistry, Chinese Academy of Sciences, P. R. China. E-mail: anmin_cao@iccas.ac.cn
dInstitute![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) for Superconducting and Electronic Materials, University of Wollongong, Wollongong, Australia
for Superconducting and Electronic Materials, University of Wollongong, Wollongong, Australia
First published on 29th May 2015
Abstract
Increasing energy and power demands have continued to stimulate the development of new electrochemical energy storage devices. Supercapacitors, well-known energy storage systems characterized by a high power density and long cycle life, have experienced a rapid progress benefiting from fast advancements in electrode materials. However, for those conventional supercapacitors assembled through a thin film preparation technique, the conductive agent and polymer binder will inevitably account for a large amount of ‘dead volume’, which should be further diminished through a better design of the supercapacitor architecture. Here, we present a comprehensive review on recent research progress on the design of integrated electrode architectures, especially the binder-free nanoarray electrodes. By means of an integration of highly-ordered active nanomaterials and a current collector, the binder-free nanoarrays can provide a larger active surface area, faster electron-transport route, easier ion diffusion and superior structural stability, thus leading to a substantially improved cycling and rate performance. This work will narrow its focus on two independent aspects of binder-free architectures: the design of electrode materials and the construction of current collectors. In addition, we also discuss and review future research directions and the remaining challenges in materials development for advanced supercapacitors.
1. Introduction
Along with the fast growth of the global economy, vast fossil-fuel consumption is causing a rapid depletion of fossil fuels and deteriorating environmental problems. It is therefore of great importance to develop highly-efficient energy storage systems so as to meet the requirements of our economy and society. Among different systems developed for energy storage,1–6 supercapacitors (SCs) have attracted worldwide attention and play a vital role in our daily lives. Their application ranges from numerous portable consumer electronics to hybrid and electric vehicles to large-scale industrial power systems.7–9 SCs are also called electrochemical capacitors (ECs) that consist of two electrodes separated by an ion permeable membrane, and an electrolyte responsible for ionic conductivity between both electrodes. Due to the high specific power (>10 kW kg−1), long life time (>105 times), and fast charge–discharge process (within seconds), SCs are considered to be one of the best devices for energy storage.6,7SCs can be divided into two major categories depending on their energy storage mechanism: electrical double layer capacitors (EDLCs) and pseudocapacitors.2 EDLCs store charge in the electrical double-layer that surrounds the electrode surface. The capacitance arises from the pure electrostatic charges in a Helmholtz double layer at the interface between the conductive electrode and the electrolyte, with its ability determined by the effective surface area of the electrode and the dielectric constant of the electrolyte. The accumulation of charge at the electrode surface is a non-faradaic process.10,11 Activated carbon, carbon aerogels, silica gels, metal supported carbon, carbon nanotubes, graphene etc., are currently widely under use as EDLC electrode materials. On the contrary, the capacitance of pseudocapacitors originates from the fast faradaic electrochemical storage with electron/charge transfer, achieved by redox reactions, intercalation, or electrosorption. Pseudocapacitors thus combine features of both capacitors and batteries. Accordingly, pseudocapacitors usually show a higher specific capacitance (300–1000 F g−1) as compared to that of carbon-based EDLC electrodes (100–250 F g−1).12 Typical pseudocapacitive materials include transition metal oxides such as RuO2, MnO2, NiO, Co3O4, V2O5, CuO, Fe2O3, WO3, etc.,13–24 and conductive polymers represented by polythiophene (PT), polypyrrole (PPy) and polyaniline (PANI).25–29 These π-conjugated conducting polymers with various heterocyclic organic compounds have shown a high gravimetric and volumetric pseudocapacitance in various non-aqueous electrolytes with operating voltages of ∼3 V.12 Although the charge storage mechanisms are different, the electrochemical performance of both EDLCs and pseudocapacitors mainly rely on the adopted electrode materials. Charge storage relies on the surface properties or depends on a thin-layer region of active materials (several tens of nanometers from the surface) by means of either adsorption/desorption of ions to form electric double layers or reversible surface/near-surface faradaic reactions.7,30,31 SCs can provide a high capacitance and high power densities owing to non-solid state ion diffusion while possessing a poor energy density (5–20 W h kg−1) caused by limited active surface areas or the partial use of entire pseudocapacitive materials.32
The key performance parameters of SCs include specific capacitance, energy density, power density, rate capability and cycling stability. To increase the energy density of SCs, a higher specific capacitance, a wide operating voltage window, and a low equivalent series resistance are highly desirable. Generally, ideal SC electrode materials are expected to possess the following characteristics: high specific surface area, specified porosity, high electronic conductivity, desirable electroactive sites, and high thermal and chemical stabilities.14,33 In future, for further applications of SCs in different research areas such as electric vehicles, SCs should be able to quickly release extraordinarily high energies (high rate capabilities), and store large quantities of electrical energy in small volumes.34–38 These stipulations will necessitate a creative progress such as using innovative electrode materials with nanostructured architectures by rational design.
Nanostructured electrode materials have shown obvious advantages over their bulk counterparts in fabricating advanced supercapacitor devices. Due to their small size, ions will have a shorter path length, which results in a faster electron-transport access at high charge–discharge rates. Additionally, the interfacial performance between the electrode material and electrolyte is enhanced, resulting in a higher ion flux when compared to that of the bulk.2–5,30,33 Despite the benefits acquired from the size reduction of the electrode materials, pseudocapacitor electrodes still have several intrinsic problems for practical application. For instance, most of the extensively studied oxides (such as MnO2, Co3O4, NiO) belong to wide band gap semiconductors or even insulators; as a result, they usually exhibit poor electrical conductivity.36–38 This is quite detrimental due to the safety concerns arising from the release of a large amount of joule heat during charge–discharge cycles. In a standard supercapacitor device assembly procedure, the electrode materials will be mixed with the binding additive and electronic conductors to form a paste, and then they are deposited onto a current collector for further electrochemical performance evaluation. The addition of an insulating polymer binder will inevitably increase the “dead volume” in electrode materials, due to the electrochemical-inertness of the binder during the charge–discharge cycles.6,31 Meanwhile, despite the obvious benefit from downsizing the electrode materials to a nanosize level, some inherent challenges such as low conductivities, slow kinetics, poor cyclability and weak mechanical properties still hinder their application and cannot be totally resolved.34,39–41 Moreover, side reactions caused by the high activity of the nano-sized electrode materials are also a disturbing issue. The reaction between electrolytes and electrodes will lead to a high level of irreversibility and thus a poor cycle life.39,42 Therefore, although the use of nanomaterials can be helpful to facilitate the reaction kinetics since the ions should much more easily penetrate into the inner side of the electrode matrix,42,43 the requirement of size reduction should also not be ignored. The electrical resistance in a solid electrode is expected to increase for nanomaterials as a result of an increased amount of grain boundaries.44
In order to address these issues and exploit high-efficiency SCs, more efforts are urgently needed on the development of novel electrode materials together with advanced electrode architectures. Nanoarrays, which are constructed from 1D or 2D nanostructures grown in a quasi-vertical alignment with their microscale lengths and nanoscale diameter/thickness, often exhibit fascinating properties, such as a uniform structure, high porosity architecture, and large surface area. The well-ordered nanoarrays have attracted wide attention for their application in SCs owing to their obvious advantages as follows:20–24,45–49 (i) the open space between the well-ordered arrays allows for easy diffusion of the electrolyte into the inner region of the electrode, and a low diffusion resistance of the electrolyte. (ii) Nanoarrays on substrates with robust adhesion can provide access to fast electron-transport to the current collector, avoiding the use of an insulating polymer binder, efficiently reducing ohmic polarization and enhancing the rate capability. (iii) The excellent structural stability of the array architecture can improve the cycling stability and rate capability, which is evident during long-term tests at high current densities. Due to the uniform structure, array nanostructures on substrates not only provide a larger electrochemically active surface area, faster electron transport and superior ion diffusion, but also possess a higher electrical conductivity and maintain a better structural mechanical stability. The design and optimization of array nanostructures are definitely important since each unit in the arrays can effectively contribute to the total capacitance if an appropriate nanostructure is selected. Ordered arrays of nanostructured materials can be synthesized on a substrate by the “bottom-up” growth approach. Since each unit (nanoarray) is directly in contact with the current collector, there is an efficient transfer of faradaic electrons from each and every nanostructure of the nanoarray to the current collector. Due to this structural integrity and high electronic conductivity, there is no need for adding extra binders or conductive additives which are used for the standard supercapacitor electrode fabrication process. By combining different kinds of materials into ordered nanoarrays, hybrid nanoarrays can be formed, which have been proved to exhibit synergistic effects. Owing to the above advantages, ordered nanoarrays are currently considered as one of the most promising candidates for future SC electrode.33,35,47
To date, there have already been some excellent reviews50,51 on advanced electrode materials for energy storage systems in the literature,8,9,12,15,32,33,45–49,52. In this article, we will review the recent progress in using rationally designed nanoarrays as electrodes for advanced SCs. This review is organized as follows: we first review carbon nanoarray materials such as carbon nanotube arrays and conducting polymer nanoarrays. After that, we specifically focus on some recent advances on transition metal oxide nanostructure arrays, including single metal oxides (Co3O4, MnO2, TiO2) and binary metal oxides such as NiCo2O4, ZnCo2O4, NiMoO4, CoMoO4, as high-performance electrodes. Next, we highlight ordered hybrid nanostructure arrays for supercapacitors with high-energy density applications. This section will be the focal point of this review because hybrid nanostructure arrays have recently received special attention due to their unique advantages,20–24,31–33,52–71 and some of our recent work is also summarized in this section. The novel support structures for loading nanoarrays, such as ordered arrays based on flexible current collectors will be the topic in the following section. Finally, we end the review with conclusions and an outlook, summarizing the nanoarray electrode materials applied in advanced SCs and pointing out several possible research trends in the electrode array architecture design for SCs.
2. Carbon and conducting polymer nanoarray electrodes
Currently, the electrodes of most commercial SCs are made of carbon materials due to their intriguing properties including low cost, easy availability, nontoxic nature, environmental friendliness and stability.72 Carbon-based SCs are operated as EDLCs. The carbon-based EDLCs have an excellent cycling stability and long service lifetime since the charge generation mechanism does not involve any faradaic reactions of the electrodes during the charge–discharge processes. Carbon nanotubes (CNTs), as the most common 1D carbon nanomaterial, have been widely used in high-performance SCs.53–60,73 Compared with CNT powder, carbon nanotube arrays possess more regular pore structures, a controllable inter-distance, specified orientation, and preferred conductive paths. More significantly, avoiding the use of polymer binders and conductive additives can effectively reduce the series resistance of the supercapacitor device. For example, Kim et al. demonstrated a facile protocol to synthesize vertically aligned CNTs on conductive carbon paper via water-assisted CVD (Chemical Vapor Deposition), and the CNT nanoarrays showed superior electrochemical characteristics for SCs.74,75 The CNTs were well attached to the carbon paper substrates with a low contact resistance. The specific capacitance of the CNT nanoarray electrode at a current density of 20 A g−1 was ca. 200 F g−1 with energy and power densities of 20 W h kg−1 and 40 kW kg−1, respectively, when measured in 1 M H2SO4 electrolyte.Template synthesis using anodized aluminum oxide (AAO) as the mold is one of the most ideal methods for the preparation of nanostructured arrays. AAO is chosen because of its facile fabrication, controllable pore-size, well-ordered pore structure, and the high density of its pores along with its amenable ability to be removed with either acidic or basic solutions.76–78 In short, one side of the alumina template can be coated with a desirable conductive material, which will serve as the current collector, and then the electroactive materials can be simultaneously or subsequently loaded into the perfectly aligned nanochannels to form well-ordered arrays. In this regard, Hahm et al. designed and developed high-power EDLCs using carbon-based three dimensional (3D) hybrid nanostructured electrodes.79 The 3D hybrid nanostructured electrodes, consisting of vertically aligned CNTs on highly porous carbon nanocups (CNCs), were synthesized by a combination of anodization and CVD techniques. The schematic of the CNT–CNC 3D hybrid fabrication approach can be found in Fig. 1. The 3D electrode-based supercapacitor showed an enhanced volumetric capacitance than that of a conventional CNC-based device by accommodating more charges in a given footprint area. However, the high cost of preparing such sophisticated nanoarrays is a hindrance for their large scale application.
 | ||
| Fig. 1 Schematic drawings and the corresponding SEM images and Raman spectra of CNC and the CNT–CNC hybrid structure. (a) Schematic drawing of CNC, (a1) SEM image of the top surface of CNC, (a2) and (a3) SEM images of the cross-sectional view, (b) schematic of vertically aligned CNT grown on a surface of CNC, (b1) top-view SEM image of vertically aligned CNTs, (b2) side view SEM image, and (b3) high-magnification SEM image of the vertically aligned CNT–CNC structure. The scale bars are 200 μm, 4 μm, and 400 nm from (1) to (3), respectively (reprinted from ref. 79 with permission). | ||
Many kinds of conducting polymers such as PT, PPy, PANI are also considered as a category of promising alternative materials for advanced SCs.80,81 The advantages of conducting polymers mainly include the ease of synthesis, a relatively high electronic/ionic conductivity and the capability to form uniform and highly porous films. Besides, conducting polymers with well-defined redox behavior can also function as good electrode materials for SCs. However, their poor mechanical stability in aqueous electrolytes usually leads to a poor cycle life, restraining further progress in real applications.82 Thus, conducting polymer nanostructure arrays would be a good choice to enhance the mechanical stability of electrodes and increase the stability during long-term cycling.
Wang et al. reported a facile one-step approach to prepare vertically aligned PANI nanowire arrays on various substrates using a galvanostatic current method (Fig. 2).83 The as-prepared large arrays of PANI nanowires had very narrow diameters and were oriented perpendicular to the substrate, which benefitted the ion diffusion when being used as a supercapacitor electrode. As shown in Fig. 2(d), the highest specific capacitance of the as-measured PANI nanowire arrays was 950 F g−1 and remained as high as 780 F g−1 at a large charge–discharge current density (40 A g−1). Wang et al. fabricated a flexible micro-supercapacitor on the pattern of a PANI nanowire array microeletrode by in situ chemical polymerization; a high volumetric capacitance of 588 F cm−3 and a considerably good rate capability were achieved. The micro-supercapacitor with nanoarray pattern makes the supercapacitor applicable as a micro-biosensor for micro/nano-devices.84
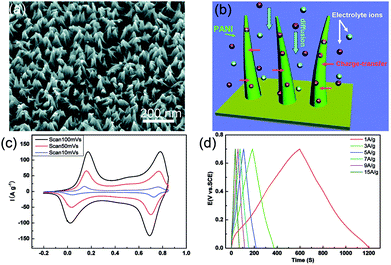 | ||
| Fig. 2 (a) SEM image of the PANI nanowire array; (b) schematic of the optimized ion diffusion path in the nanowire arrays; (c) cyclic voltammetry plots at different scan rates; (d) typical galvanostatic charge–discharge curves at several current densities. Reprinted from ref. 83 with permission. | ||
A template-free approach to fabricate a large area of length-controllable and well-oriented polypyrrole nanowire arrays was also reported, and the PPy nanowire arrays exhibited an enhanced capacitance value compared with those of disordered nanowire networks or compact films.85 A layer of Au nanoislands, that function as pseudo-nuclei, could activate the nucleation of PPy and then these active nucleation centers would minimize the interfacial energy barrier for the following growth of PPy on the substrate. The nanowire arrays exhibited a remarkable capacitance of 566 F g−1 and retained 70% of their initial capacitance after hundreds of charge–discharge cycles, which is much better than those of disordered nanowire networks and conventional films.
3. Metal oxide nanoarray electrodes
Metal oxides have long been studied as potential electrode materials for SCs due to the ease of large-scale fabrication and their many redox reactions, which contribute to the high specific capacitance.86–88 In particular, the capacitance of transition metal oxides generated via the conversion mechanism is high. In addition, some metal oxides such as iron and manganese oxides are abundant in nature and thus low-cost. The size, morphology and orientation of metal oxide nanostructures can also be easily tuned, which makes it possible to systematically investigate the structure–electrochemical property relationship. However, it is difficult to directly use metal oxides as electrodes in supercapacitor prototypes due to the following drawbacks: firstly, the conductivity of most metal oxides, except for RuO2, is very low. The poor conductivity of metal oxides increases both the sheet resistance and the charge transfer resistance of the electrode and especially causes a large IR loss at a high current density. Thus, the power density and rate capability are far from satisfying. Secondly, the strain developed in pure metal oxide during the charge–discharge processes causes cracking and other structural degradation of the electrode, leading to a poor long-term stability. Thirdly, the surface area, the pore distribution as well as the porosity are difficult to tailor in metal oxides. It is logical to develop advanced electrode architectures to mitigate the shortcomings of transition metal oxides. Taking NiO as an example, vertically aligned NiO single-crystalline nanoplatelet arrays directly grown on a fluorine-doped tin oxide (FTO) substrate by a simple hydrothermal method were fabricated by Wu’s group.89 They found that the ammonia and persulfate concentrations were the key parameters to control the morphology of the nanoarray film. It exhibited a high specific capacitance, prompt charge–discharge rate and good stability of the cycling performance. The aligned single-crystalline NiO nanoplatelet array was beneficial to the charge transfer in the electrode and to the ion transport in the solution during the redox reaction. Lu et al. demonstrated that hydrogenation significantly improved the electrochemical performance of TiO2 nanotube arrays (NTAs) as supercapacitor electrodes, as illustrated in Fig. 3. TiO2 NTAs hydrogenated at 400 °C delivered a specific capacitance of 3.24 mF cm−2 at a scan rate of 100 mV s−1 with an energy density of 0.8 mW h cm−2 and a power density of 17.5 mW cm−2. Importantly, the H–TiO2 electrode exhibited excellent long-term stability with only 3.1% reduction in capacitance after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles.22 According to Fig. 3(d) and (e), in comparison to the untreated TiO2 and air–TiO2 samples, the H–TiO2 sample delivered an obvious pseudocapacitive characteristic, which can be attributed to the oxidation/reduction of surface hydroxyl groups.
000 cycles.22 According to Fig. 3(d) and (e), in comparison to the untreated TiO2 and air–TiO2 samples, the H–TiO2 sample delivered an obvious pseudocapacitive characteristic, which can be attributed to the oxidation/reduction of surface hydroxyl groups.
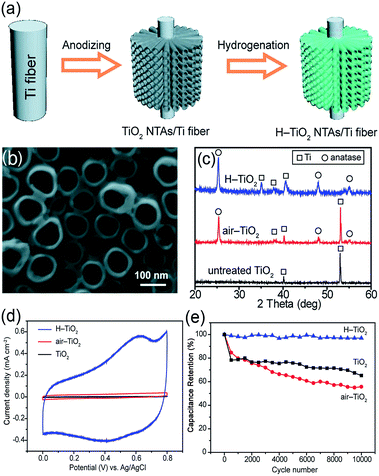 | ||
Fig. 3 (a) Schematic diagram showing the fabrication of H–TiO2 NTAs; (b) SEM image of H–TiO2 NTAs. (c) XRD spectra collected from untreated TiO2, air–TiO2, and H–TiO2 NTAs; (d) CV curves of untreated TiO2, air–TiO2, and H–TiO2 NTAs obtained at a scan rate of 100 mV s−1; (e) cycle performance of the TiO2 samples measured at a scan rate of 100 mV s−1 for 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. Reprinted from ref. 23 with permission. 000 cycles. Reprinted from ref. 23 with permission. | ||
Gao et al. prepared Co3O4 nanowire arrays freely standing on nickel foam via template-free growth followed by thermal treatment at 300 °C in air.90 The results showed that nanowires with diameters of around 250 nm were formed by nanoplatelets roughly packed layer by layer, and densely covered the nickel foam substrate. The Co3O4 nanowires displayed a specific capacitance of 746 F g−1 at a current density of 5 mA cm−2. The capacitance loss was less than 15% after 500 charge–discharge cycles. It is worth noting that the selection of a suitable substrate could significantly enhance the performance of transition metal oxide nanoarrays. Xia et al. compared the supercapacitive behavior of Co3O4/NiO nanowire arrays on various substrates. They found that Co3O4/NiO nanowire arrays grown on nickel foam exhibit enhanced performance than that of arrays grown on transparent conducting glass and nickel foil.61
MnO2 as an important metal oxide for SCs has been designed to be nanoarray-structured to improve its poor electrical conductivity. Luo et al. synthesized self-assembled well-ordered whisker-like MnO2 arrays on carbon fiber paper (MOWAs) via a simple in situ redox replacement reaction between potassium permanganate and carbon fiber paper (CFP) without any other oxidant or reductant addition.59 CFP served as a sacrificial reductant and converts aqueous permanganate to insoluble MnO2 in this reaction; at the same time, CFP functions as a substrate material and guarantees MnO2 deposition on the surface. The high-performance hybrid composites resulted from a synergistic effect of the large surface area and high degree of ordering of the ultrathin layer of the MnO2 nanowhisker arrays combined with the flexible CFP substrate, and can offer great potential for large-scale energy storage device applications.
Binary metal oxides have been reported to exhibit better performances than single component oxides due to their achievable oxidation states and high electrical conductivities such as NiCo2O4,87,88,91,92 Zn2SnO4,93 NiMoO4,71 CoMoO4,69 and ZnCo2O4.94 Binary metal oxides have been considered as promising, effective and scalable alternatives, since they offer many advantages such as low cost, abundance and environmental friendliness. NiCo2O4 has recently been investigated as a high-performance electrode material for SCs because of its better electrical conductivity and higher electrochemical activity compared with those of nickel oxide (NiO) or cobalt oxide (Co3O4). There have been several reports on the synthesis and electrochemical performance evaluation of nanostructured NiCo2O4 materials.87,88 Recently, Lou’s group has reported the preparation of single-crystalline nanoneedle arrays of NiCo2O4 on conductive substrates through a simple solution method together with a post-annealing treatment.91 The NiCo2O4–Ni foam electrode exhibited a greatly improved electrochemical performance with a very high capacitance and excellent cycling stability. In another work, interconnected NiCo2O4 mesoporous nanosheets were grown on various conductive substrates through a similar strategy (Fig. 4).92 The NiCo2O4 mesoporous nanosheets supported on Ni foam directly served as binder- and conductive-agent-free electrodes for SCs. Benefiting from the rational structural features, the NiCo2O4 nanosheets–Ni foam electrode had a capacitance of as high as 3.51 F cm−2 at a discharge current density of 1.8 mA cm−2. The areal capacitance reached a high value of 2.09 F cm−2 when a discharge current density of 8.5 mA cm−2 was applied, and the capacitance gradually decreased to 1.95 F cm−2 after 3000 cycles, resulting in an overall capacitance loss of only 6.7% (Fig. 4(d)). The excellent electrochemical performance could be derived from the following structural features: firstly, the mesoporous feature of the NiCo2O4 nanosheets largely increased the amount of electroactive sites. Secondly, the highly porous film structure formed by the interconnected nanosheets greatly facilitated the transport of electrolyte. Thirdly, the direct growth of interconnected two-dimensional nanosheets on a conductive substrate could ensure good mechanical adhesion, and more importantly good electrical contact with the conductive substrate that also served as the current collector in such highly integrated electrodes. For the same reason, a hierarchical ZnCo2O4/nickel foam architecture was synthesized via simple scalable solution approach by Liu et al. The ZnCo2O4/nickel foam architectures exhibited an outstanding electrochemical performance as SC electrodes with a high specific capacitance (1400 F g−1 at 1 A g−1), excellent rate capability (72.5% capacity retention at 20 A g−1), and good cycling stability (only 3% loss after 1000 cycles at 6 A g−1).94 All-solid-state SCs were also fabricated by assembling two pieces of the ZnCo2O4-based electrodes, and exhibited a superior electrochemical performance in terms of high specific capacitance and long cycling stability.
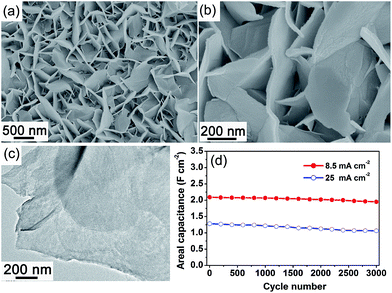 | ||
| Fig. 4 (a) and (b) SEM images of the NiCo2O4 nanosheets on Ni foam; (c) TEM image of the NiCo2O4 nanosheets scratched from Ni foam; (d) the capacitance as a function of the cycle number at constant current densities of 8.5 and 25 mA cm−2. Reprinted from ref. 92 with permission. | ||
Metal molybdates such as CoMoO4,69 NiMoO4,71 and MnMoO4,70 have demonstrated vastly improved performances over those of single component oxides. Our group has reported a CoMoO4 nanoarray with a high specific capacitances of 1.26 F cm−2 at a discharge current density of 4 mA cm−2 and 0.78 F cm−2 at 32 mA cm−2 with an excellent cycling ability (79.5% of the initial specific capacitance remained after 4000 cycles).69 In addition, to confirm the advantages of the array structure which was grown directly on a current collector, control experiments were performed by pasting CoMoO4 powder onto Ni foam. The superior electrochemical performance of CoMoO4 is mainly attributed to the advantageous structure of the electrode. The thin NPs (nanoplates) give rise to a high surface area, provide more active sites for faradic reactions and improve the charge collection efficiency of the active materials.
4. Ordered hybrid nanostructure array electrodes
Although several strategies have been explored to optimize the electrochemical performance of nanostructure arrays, nearly all of them follow the same underlying principle, that is the design and fabrication of hybrid nanostructure arrays.95–98 The hybrid nanostructure arrays possess all advantages of ordered single-phase arrays, and further bring along some unique merits of hybrid composites. For example, in carbon–metal oxide hybrid nanoarray electrodes, the carbon nanostructures not only serve as the physical support of metal oxides, but also provide channels for charge transport. The high electronic conductivity of carbon nanostructures is beneficial to the rate capability and power density of SC electrodes at a large charge–discharge current. Pseudocapacitive materials will only be able to fully utilize the capacitance from the reversible redox reactions on the electrode surface. Therefore, composites incorporating a porous carbon substrate with pseudocapacitive materials suggest a potential breakthrough for a new generation of SCs. Metal oxides as the dominant contributor for specific capacitance and power, endow a high specific capacitance and high energy density for carbon–metal oxide nanoarray electrodes. A synergistic effect could be expected and the materials cost can be reduced. The compositional constituent, microstructure and physical properties of metal oxide–carbon nanoarrays govern the performance of the supercapacitor electrodes. The key to achieve high power and energy density hybrid nanoarray SCs with a long cycle life is to explore novel electrode material systems with a rational design and proper selection of material components, tuning the morphology, and controlling the size to obtain an excellent ion conductivity and electrochemical stability. Then, we will review ordered hybrid nanostructure array electrodes in the following section.4.1 Hybrid array electrodes based on carbon & metal oxides
CNTs, which are well-known for their high conductivity and unique mechanical properties, have been extensively studied for EDLC applications within the past decade.72–74 It is well accepted that CNTs are good candidates for high power electrodes due to their high mechanical resilience and open tubular network, making them a good support for other active materials. Owing to the above unique merits, CNT-based nanoarrays have been intensively investigated for SCs by Gu’s group. The manganese oxide/CNTA composites possessed a high rate capability (50.8% capacity retention at 77 A g−1), high specific capacitances (199 F g−1 and 305 F cm−3) and a long cycle life (only 3% capacity loss after 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 charge–discharge cycles).99 Such an intriguing capacitive performance can be mainly attributed to the superior conducting network consisting of CNTs, as well as the pseudocapacitive behavior of the well-dispersed active materials. Cui et al. also deposited Mn3O4 nanoparticles within a highly dense, millimeter long carbon nanotube array (CNTA) by the dip-casting method using non-aqueous solutions.100 The hydrophilic Mn3O4/CNTA composite electrodes presented an improved performance for SCs, compared with those of the as-grown CNTA electrodes. The maximum specific capacitance of the Mn3O4/CNTA composite electrode was found to be 143 F g−1. The as-deposited Mn3O4 nanoparticles demonstrated a superior specific capacitance and rate capacity because of their nanosize and excellent nanostructured scaffold, the millimeter-long CNTA.
000 charge–discharge cycles).99 Such an intriguing capacitive performance can be mainly attributed to the superior conducting network consisting of CNTs, as well as the pseudocapacitive behavior of the well-dispersed active materials. Cui et al. also deposited Mn3O4 nanoparticles within a highly dense, millimeter long carbon nanotube array (CNTA) by the dip-casting method using non-aqueous solutions.100 The hydrophilic Mn3O4/CNTA composite electrodes presented an improved performance for SCs, compared with those of the as-grown CNTA electrodes. The maximum specific capacitance of the Mn3O4/CNTA composite electrode was found to be 143 F g−1. The as-deposited Mn3O4 nanoparticles demonstrated a superior specific capacitance and rate capacity because of their nanosize and excellent nanostructured scaffold, the millimeter-long CNTA.
Recently, graphene as a novel carbon material has been promising in SC application, especially three-dimensional (3D) graphene which has a superior electrical conductivity (which provides electronic “superhighways”). The electrons can be transferred more efficiently during charge–discharge processes at large current densities through 3D graphene, and therefore lead to a significant improvement in the specific capacitance. Recently, Yu et al. have presented a simple and cost-effective synthesis strategy for the growth of novel nanohoneycomb (NHC)-like CoMoO4 nanosheets on a 3D graphene framework as the electrode material for SCs, as illustrated in Fig. 5. The electrochemical measurements of the NHC-like strongly coupled CoMoO4/3D graphene hybrid electrodes exhibited an excellent specific capacitance and superior long-term cycle stability, with a specific capacitance retention rate of 96.36% after 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at a large charge–discharge current density (400 A g−1).101 The 3D graphene network structure with excellent mechanical strength and flexibility, combined with the nanohoneycomb-like-structured CoMoO4 formed a stable architecture and could improve the electrode stability during charge–discharge processes at a large current density.
000 cycles at a large charge–discharge current density (400 A g−1).101 The 3D graphene network structure with excellent mechanical strength and flexibility, combined with the nanohoneycomb-like-structured CoMoO4 formed a stable architecture and could improve the electrode stability during charge–discharge processes at a large current density.
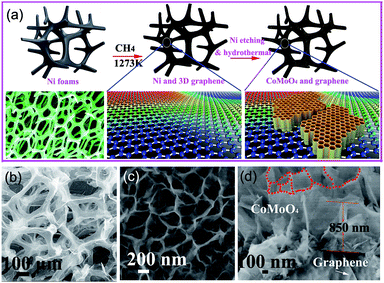 | ||
| Fig. 5 (a) Typical synthesis procedure of the honeycomb (NHC)-like CoMoO4–3D graphene hybrid electrodes; (b) SEM image of 3D graphene; (c) SEM image of the NHC-like strongly coupled CoMoO4–3D graphene hybrid; (d) cross-section SEM image of the NHC-like CoMoO4–3D graphene hybrid. Reprinted from ref. 101 with permission. | ||
4.2 Hybrid array electrodes based on polymers & metal oxides
Hybrid array electrodes, together with the synergistic effects of different pseudocapacitive materials, can also enhance the supercapacitor performance. Conducting polymers and metal oxides are both ideal pseudocapacitive materials with a high capacitance. The hybrid nanoarrays would combine the good stability of metal oxides with the good electrical conductivity of polymers and complement their own characteristic.12,71 For example, Naoi et al. reported MnO2/poly(3,4-ethylenedioxythiophene) (PEDOT) co-axial nanowires synthesized by a one-step electrochemical deposition process using a porous alumina template (Fig. 6(a)–(e)).25 In this composite, the MnO2 core is utilized for its high energy density, while the PEDOT shell is applied for its high conductivity, and porous and flexible nature. The PEDOT shell facilitates electron transportation and ion diffusion into the energy dense MnO2 core and protects the core from structural collapse and degradation. The hybrid nanoarrays resulted in a synergetic hybrid array that had very high specific capacitances at high current densities as opposed to those of pure MnO2 nanowires and PEDOT nanowires (Fig. 6(f)).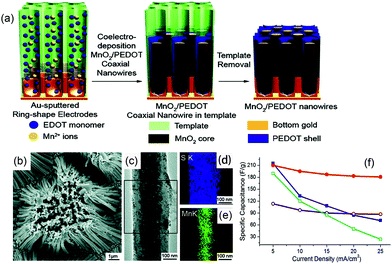 | ||
| Fig. 6 (a) Growth mechanism of the MnO2/PEDOT coaxial nanowires; (b) SEM image of the MnO2/PEDOT coaxial nanowires; (c) TEM image of a single coaxial nanowire; (d and e) EDS maps of S and Mn from the boxed area in (c); (f) rate performance (red) of the coaxial MnO2/PEDOT nanowires via electrodeposition. Reprinted from ref. 25 with permission. | ||
Recently, there are some reports about hybrid nanoarrays of metal oxides and PPy. Wang et al. explored a novel ZnO nanorod array template-assisted electrodeposition route to synthesize large-scale single-walled PPy nanotube arrays (NTAs) and multi-walled MnO2/PPy/MnO2 NTAs.102 The structural features of the nanotubes, such as the external and inner diameters, wall thicknesses, and lengths, can be well controlled by adjusting the diameters and lengths of the ZnO nanorods together with the deposition time. The synthesized hybrid MnO2/PPy/MnO2 triple-walled nanotube arrays as electrodes showed high supercapacitive properties, excellent long-term cycling stability, and high energy and power densities. The PPy layers in MnO2/PPy/MnO2 provide reliable electrical connections to the MnO2 shells and uniquely serve as highly conductive cores to support the redox reactions in the active two-double MnO2 shells with a highly electrolytic and accessible surface area. The fabricated multi-walled NTAs allow highly efficient utilization of the electrode materials with facilitated transports of ions and electrons.
It is noteworthy that the hybrid array can be fabricated by a template-free method. For instance, Liu’s group fabricated an asymmetric supercapacitor electrode composed of a well-aligned CoO nanowire array grown on 3D Ni foam with PPy uniformly immobilized onto or firmly anchored to each nanowire surface to boost the pseudocapacitive performance (Fig. 7(a) and (b)).53 The asymmetric supercapacitor device fabricated using the hybrid array as the positive electrode and an activated carbon film as the negative electrode, demonstrating a high energy density (43.5 W h kg−1), high power density (5500 W kg−1 at 11.8 W h kg−1) and outstanding cycling stability (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times) (Fig. 7(d) and (f)). Furthermore, two such asymmetric SCs connected in series can efficiently power a red LED, lasting for 1 h (Fig. 7(e)). The electrode architecture takes advantage of the specific superior electrochemical activity from both CoO and PPy, such as the high electronic conductivity of PPy and the short ion diffusion pathway in ordered CoO mesoporous nanowires. These merits together with the elegant synergy between CoO and PPy lead to a higher specific capacitance compared with that of pure CoO.
000 times) (Fig. 7(d) and (f)). Furthermore, two such asymmetric SCs connected in series can efficiently power a red LED, lasting for 1 h (Fig. 7(e)). The electrode architecture takes advantage of the specific superior electrochemical activity from both CoO and PPy, such as the high electronic conductivity of PPy and the short ion diffusion pathway in ordered CoO mesoporous nanowires. These merits together with the elegant synergy between CoO and PPy lead to a higher specific capacitance compared with that of pure CoO.
 | ||
| Fig. 7 (a) SEM image of the hybrid nanowire electrode; (b) HRTEM image of the surface of individual CoO@PPy hybrid nanowires; (c) CV curve comparison of the optimized hybrid electrode and the pristine CoO electrode; (d) volumetric energies and powder densities of our supercapacitor compared with other data. (e) Images of the red LED at different stages; powered by the 10 s charged SC; (f) cycling stability of the two-electrode device. Reprinted from ref. 53 with permission. | ||
4.3 Hybrid array electrodes based on carbon & polymers
The hybrid array electrodes of carbon and conducting polymers combine the advantages of both materials. Conducting polymers provide a high pseudocapacitance value, while nanostructured carbon materials act as a framework that helps the conducting polymers to sustain from strains during the charge–discharge cycling process. Currently, the research in this field focuses on designing hybrid structures, improving the interface between carbon and the conducting polymers, and tuning the chemical structure of the conducting polymer for high electrochemical activities.12 Zhang et al. reported the use of a carbon nanotube array (CNTA) framework, which was directly grown on the current collector (Ta foil) as the support to construct PANI/CNTA composite electrodes with hierarchical porous structures.103 The electrochemical results indicated that a very high specific capacitance of 1030 F g−1 was achieved for the PANI/CNTA composites with a superior rate capability (95% capacity retention at 118 A g−1), and high cycling stability (only 5.5% capacity loss after 5000 cycles) in 1 M H2SO4 electrolyte. Such an intriguing capacitive performance can mainly be attributed to the superior conducting network provided by CNTs, as well as the high pseudocapacitive charge of the well-dispersed PANI. In another work, a CNTs@PANI composite yarn based supercapacitor fabricated by Wang et al. showed a capacitance of 38 mF cm−2 while the areal capacitance of the pure CNT yarn-based supercapacitor was only ca. 2.3 mF cm−2, representing a 16-fold improvement. The improvement in capacitance can be attributed to the ordered PANI nanowire arrays which are recognized as a high-performance pseudocapacitance material.104The conducting polymer nanoarrays can also be constructed on graphene powder. Xu et al. developed a facile approach to prepare PANI nanowire arrays vertically aligned on graphene oxide nanosheets, as shown in Fig. 8. The hierarchical nanocomposite, when applied as a supercapacitor electrode, possessed a higher electrochemical capacitance value (555 F g−1) than that of randomly connected PANI nanowires obtained under the same conditions (298 F g−1), and exhibited good cycling stability (92% capacity retention after 2000 cycles).105
 | ||
| Fig. 8 Hierarchical nanostructures mounted on graphene oxide nanosheets; (a and b) SEM images of the PANI–GO nanocomposites at different magnifications; (c) schematic diagram of the nucleation and growth mechanism: heterogeneous nucleation (a) and homogeneous nucleation (b); (d) CV curves of pristine GO, randomly connected PANI nanowires, and PANI–GO; (e) cyclic stability of pure PANI and the PANI–GO nanocomposites. Reproduced with permission. Reprinted from ref. 105 with permission. | ||
4.4 Hybrid array electrodes based on metal oxides/metal hydroxides (oxides)
Hybrid electrodes are emerging as an efficient strategy for the development of a diverse range of next-generation SCs. Currently, despite some significant advances already achieved, the research and development of hybrid arrays are still in their early stages, therefore more efforts are contributed to advanced structural designs, and reliable hybrid nanoarray electrode fabrication methods are highly required.32,52 For hybrid metal oxide nanoarray electrodes, several highlights are noteworthy. First, both the core and shell components are good pseudocapacitive metal oxides. The nanostructured core and shell can undergo redox reactions with anions and cations from the electrolyte, respectively, synergistically contributing to the high capacitance value.31,57 Second, the hybrid nanomaterials are directly and robustly attached to the current collector, which avoids the use of binders and substantially reduces the “dead volume” in the electrode.Fan’s group has done a lot of excellent work on advanced SCs based on hybrid array electrodes.57,106 Xia et al. reported the fabrication of porous metal hydroxide nanosheets on a preformed nanowire scaffold using the fast and well-controllable electrodeposition method.106 Co(OH)2 nanosheets were electrochemically deposited on Co3O4 core nanowires to form core–shell arrays, as shown in Fig. 9(a)–(c). The Co3O4/Co(OH)2 core–shell nanowire arrays were evaluated as a supercapacitor cathode material that exhibited high specific capacitances of 1095 F g−1 at 1 A g−1 and 812 F g−1 at 40 A g−1, as shown in Fig. 9(d). In the electrode design, all the desired functions of each constituent are effectively utilized, producing a so-called “strong synergistic effect”. To prove this concept, Liu et al. chose MnO2 as the model shell and Co3O4 as the core material (Fig. 9(e) and (f)). The Co3O4@MnO2 hybrid nanowire array presented a high specific areal capacitance (4 to 10-fold increase depending on current rates) with respect to that of the pristine Co3O4 array, as shown in Fig. 9(g).57
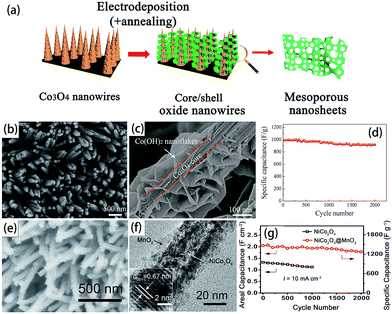 | ||
| Fig. 9 (a) Schematic presentation of the porous hydroxide nanosheets formed by electrodeposition on preformed nanowire arrays. (b) and (c) SEM images of the Co3O4/Co(OH)2 core–shell nanowire arrays; (d) cycling performance of Co3O4/Co(OH)2 at 2 A g−1. Reprinted from ref. 106 with permission. (e) Hierarchical NiCo2O4@MnO2 core–shell NW arrays grown on Ni foam; (f) TEM image of the NiCo2O4@MnO2 core–shell heterostructured NWs scratched from Ni foam; (g) cycling performance of the NiCo2O4 NW arrays and hierarchical NiCo2O4@MnO2 core–shell NW arrays. Reprinted from ref. 64 with permission. | ||
Another typical example is the fabrication of a novel hybrid nanostructure of porous CoO nanowires@ultra-thin nickel hydroxide nitrate nanoflakes (Ni3(NO3)2(OH)4) on a 3D nickel foam substrate. CoO nanowires pre-grown on the nickel foam are able to serve as the “initially formed oxide nanostructure arrays” and react with the introduced Ni(NO3)2 aqueous solution. The above-mentioned hybrid structure integrates the merits of the two active components which exhibit a good electrochemical stability and high-rate performance compared to those of the pure CoO nanowires or the physical mixture of the two components.66 Yu et al. also successfully synthesized hierarchical NiCo2O4@MnO2 core–shell heterostructured nanowire arrays on nickel foam (Fig. 9). As shown in Fig. 9(g), it is clear that both the ASC (area specific capacitance) and cycling stability are largely enhanced in the core–shell NW array electrode. The ASC increased from 1.33 F cm−2 for the bare NiCo2O4 NW arrays to 2.05 F cm−2 for the core–shell NW arrays.
Following a similar concept, another two excellent works of hybrid metal oxide-based nanoarrays, a tubular array of Ni(OH)2 nanosheet@Fe2O3 nanowire hybrid composite arrays107 and NiCo2O4@Co3O4 core–shell nanoforest arrays,54 have been reported. In the former work, the Ni(OH)2@α-Fe2O3 hybrid composites exhibited an excellent rate capability with a specific energy of 22.8 W h kg−1 and a specific power of 16.4 kW kg−1 at a current density of 54.6 A g−1, and an excellent long-term cycling stability (kept over 85.7% of the initial specific capacitance after 5000 cycles). For the latter, nanoforests of hierarchical Co3O4@NiCo2O4 nanowire arrays performed with high areal capacitances of 2.04 F cm−2 at a scan rate of 5 mV s−1 and 0.79 F cm−2 (almost 2.5 times as high as that of pristine Co3O4) even at 30 mA cm−2 after 6000 cycles with varying current densities.
5. Novel support structures for loading nanoarrays
5.1 Current collector-based ordered arrays
SCs based on pseudocapacitive transition-metal oxides are predicted to have a high capacitance while also being inexpensive and environmentally friendly. However, as discussed above, most pseudocapacitive transition-metal oxides have poor electrical conductivity, such as MnO2 with a conductance of 10−5 to 10−6 S cm−1.31 It is difficult to increase the electrical conductivity of metal oxides fundamentally by fabricating nanoarrays. In addition, the faradic redox reactions only happen at the surface of the active materials. So, great effort has been made to fabricate nanostructured pseudocapacitive materials, aiming to enlarge the specific surface area and shorten the electron conduction length. Another approach is to load pseudocapacitive materials onto conductive ordered array supports with a large specific surface area. The array nanostructured current collectors have a much larger surface area than that of the planar bulky ones. This enables loading of a dramatically increased amount of active materials, thus improving the energy density per unit area. More importantly, they are capable of providing both efficient pathways for ion and electron transport through the entire electrode architecture. Also, good mechanical robustness is another feature of nanostructured current collectors, which ensures that the SCs can retain their capacitance after many cycles even at a high rate.108–111When the current collectors are transformed into arrays, the electrode materials can be subsequently coated on these nanoarrays to form composite electrodes. For example, the Ni3(NO3)2(OH)4–ZnO hybrid nanowire array was demonstrated to be a good supercapacitor electrode, which could be fully charged and discharged within a few seconds.96 The hybrid array showed a specific capacitance of as high as 1310 F g−1 at 15.7 A g−1, nearly approaching the theoretical capacitance of nickel hydroxide nitrate. In addition to metal oxides, Si nanowire array was also employed as a current collector for pseudocapacitive materials such as NiO.112 Highly ordered NiO-coated Si nanowire arrays were fabricated as electrode materials for SCs via depositing Ni on electroless-etched Si nanowires. The specific capacity reached 787.5 F g−1 at a discharge current of 2.5 mA and decreased slightly with 4.039% loss after 500 cycles. Owing to its favorable electrochemical performance, this ordered hybrid array nanostructure is a promising electrode material for future commercial supercapacitor (Fig. 10).
 | ||
| Fig. 10 (a) and (b) show SEM images of Co3O4 nanorod arrays and Co9S8 nanorod arrays, respectively; (c) schematic illustration of the as-assembled ASC; (d) CV curves collected at the scan rate of 100 mV s−1 for the solid-state ASC device under normal, bent, and twisted conditions. Insets are the device photographs under different test conditions; (e) rate capability of liquid ASC and solid ASC at different current densities; (f) Ragone plots of LASC and SASC devices. Reprinted from ref. 146 with permission. | ||
However, the space between the subunits, the diameter of the current electrode and the volume of the electrode material for these array current collectors are worth noting. A small diameter would possibly lead to the collapse of the electrode after sufficient active material loading, while too big a size of subunits would occupy too much of the valuable volume. Furthermore, the ratio of the space taken by the electrode material to the space taken by the electrolyte needs to be carefully considered. Electrode materials require enough loading as to maximize the volumetric energy density of the electrode while not taking over the space needed for the electrolyte, which could cause depletion of ions in the nanosized channels. Hence, optimizing the size of subunits in the current collector represents a significant challenge.32,34
5.2 Three-dimensional graphene current collectors
More recently, the development of SCs has focused on the use of graphene, due to its excellent electrical and mechanical properties, chemical stability, high specific surface area of up to 2675 m2 g−1, and feasibility for large-scale production graphene.113–115 So far, most results on graphene-based nanocomposites have been achieved by incorporating guest nanoparticles onto 2D graphene sheets. However, those structures suffer from graphene aggregation, which causes inferior ionic accessibility and thus modest improvement in the cell performance. Obviously, the disordered nanocomposite films containing the aggregated graphene make it difficult for ions to gain access to the electrode surfaces and thus become a scientific and technical challenge.Recent reports and reviews have described an electrode construction consisting of three-dimensional interpenetrating structures that could provide a good solution to the issue of the poor ionic and electronic transport in electrode materials, thereby resulting in high-performance devices.116,117 In particular, 3D graphene has continuously interconnected macroporous structures, with a large surface area, low mass density, and high electrical conductivity,118,119 and the 3D porous structure is ideal to serve as a scaffold for the fabrication of monolithic nanoarrays and hybrid electrodes.120,121 All previous experimental results show that inorganic electrochemically active nanomaterials that directly nucleate and grow on 3D graphene exhibited superior electrochemical performance than those of graphene-active nanomaterials and single-structured materials.122,123 Therefore, 3D graphene as a novel current collector has a future in SCs. Currently, the synthesis of 3D graphene mainly proceeds via two strategies: (i) by fabricating sandwich-type structures by the introduction of “spacer” phases (e.g., carbon nanotubes, nanoparticles, and even “water molecules”),124–126 and (ii) by forming 3D macroporous structures (e.g., foams, aerogels, sponges, hydrogels, and networks) by the template-directed deposition or controlled assembly of graphene.113,127,128 Choi et al. employed a replicating and embossing technique to fabricate 3D macroporous graphene films using polystyrene (PS) colloidal particles as sacrificial templates.129 The unique electrode structure boosted ion and electron movements in the electrochemical processes and enabled the porous graphene films to serve as a 3D skeleton for combining the graphene films with MnO2.
Chemical vapor deposition (CVD) is a common method to produce 3D graphene with high quality. In general, the preparation of novel 3D graphene networks uses Ni foam as a sacrificial template in a facile CVD process with the carbon source. The Ni foam template would be removed by immersing it in PMMA and an etchant solution, at a time. After removal of the Ni foam, the 3D graphene network can be obtained. The 3D graphene networks are excellent substrates for the construction of 3D graphene/metal oxide composites, which can be used as supercapacitor electrodes. As a proof of concept, Cao et al.130 prepared 3D graphene in a facile CVD process with ethanol as the carbon source, and NiO has been electrochemically deposited on the 3D graphene networks. The unique 3D porous structure of the graphene network with a large specific surface area enables the rapid access of electrolyte ions to the NiO surface. The obtained NiO/3D graphene composite exhibits a high specific capacitance of ≈816 F g −1 at a scan rate of 5 mV s−1 and a stable cycling performance without any decrease in its specific capacitance after 2000 cycles. Dong et al. also fabricated cobalt oxide nanowires via a simple hydrothermal procedure synthesized in situ on 3D graphene foam grown by CVD. The Co3O4/3D graphene is capable of delivering a high specific capacitance of ∼1100 F g−1 at a current density of 10 A g−1 with an excellent cycling stability.122
5.3 Flexible current collectors for loading nanoarrays
Flexible power sources attract a growing interest driven by the need for modern electronic systems. In practical applications, a flexible power device must be capable of accommodating frequent complex strains (e.g., bending, twisting, deforming, etc.) whilst retaining its continuous energy supply.93,131–133 In this regard, the use of current collectors with soft, bendable and elastic properties is the prerequisite for making flexible power devices. Flexible current collectors can be realized by pre-coating various flexible scaffolds with conductive components. Currently, plastics such as polyethylene terephthalate (PET) and polydimethylsiloxane (PDMS), are the predominant scaffold materials by virtue of their low cost, good processability and flexibility. For example, Yang et al. demonstrated a flexible three-dimensional nanoporous NiF2-dominant layer on polyethylene terephthalate. The nanoporous layer itself can be freestanding without adding any supporting carbon material or conducting polymer. By assembling the nanoporous layer into two-electrode symmetric devices, the inorganic material delivers a battery-like thin-film supercapacitive performance with a maximum capacitance of 66 mF cm−2 (733 F cm−3 or 358 F g−1), an energy density of 384 W h kg−1, and a power density of 112 kW kg−1.134Papers, polymers and textiles (e.g., cotton, silk, leather, etc.) have also been proven to be potential candidates for making attractive bendable powering devices.135–139 Li and co-workers converted cotton T-shirt textiles into activated carbon textiles (ACTs) for energy storage applications, which the broaden flexible platform range in our daily life.140 Following the same concept, a promising scaffold with many advantages for flexible SCs is commercial carbon cloth (CC). CC is not only electronically conductive and mechanically flexible, but also light-weight, highly porous, and chemically stable in the electrolyte. In addition, CC can be easily rolled, packaged and further processed to fulfill the requirements of flexible electronics or wearable devices.59,141–144 When CC is used directly as the current collector, a broad spectrum of metal oxide nanomaterials might be deposited for electrochemical energy storage. A recent example is the fabrication of a flexible nanohybrid by coating MnO2 thin films onto relatively electrically conductive Zn2SnO4 nanowires grown radically on CC fibers.93 Another excellent example are large-area manganese oxide nanorods (MONRAs) on carbon fabric as flexible SCs by Yu and coworkers.145 Electrochemical measurements demonstrated that MONRAs exhibited a high capacitance (678 F g−1 at a current density of 0.3 A g−1) with a high flexibility and excellent cycle performance (less than 3% capacitance loss after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles). Recently, Shen’s group has successfully fabricated flexible asymmetric supercapacitors (ASCs) based on acicular Co9S8 nanorod arrays as positive materials and Co3O4@RuO2 nanosheet arrays as negative materials on woven carbon fabrics. Carbon cloth was selected as both the substrate and the current collector for its good conductivity, high flexibility, good physical strength, and lightweight architecture. Both aqueous KOH solutions and polyvinyl alcohol (PVA)/KOH were employed as the electrolyte for electrochemical measurements. The as-fabricated ASCs exhibited superior electrochemical performance with an energy density of 1.21 mW h cm−3 at a power density of 13.29 W cm−3 in the aqueous electrolyte and an energy density of 1.44 mW h cm−3 at a power density of 0.89 W cm−3 in the solid-state electrolyte.146
000 cycles). Recently, Shen’s group has successfully fabricated flexible asymmetric supercapacitors (ASCs) based on acicular Co9S8 nanorod arrays as positive materials and Co3O4@RuO2 nanosheet arrays as negative materials on woven carbon fabrics. Carbon cloth was selected as both the substrate and the current collector for its good conductivity, high flexibility, good physical strength, and lightweight architecture. Both aqueous KOH solutions and polyvinyl alcohol (PVA)/KOH were employed as the electrolyte for electrochemical measurements. The as-fabricated ASCs exhibited superior electrochemical performance with an energy density of 1.21 mW h cm−3 at a power density of 13.29 W cm−3 in the aqueous electrolyte and an energy density of 1.44 mW h cm−3 at a power density of 0.89 W cm−3 in the solid-state electrolyte.146
6. Conclusion
Currently, the primary challenges for SCs are their limited energy density while they have a relatively high cost, which have been the major focus points of numerous researches in the field of SCs. Despite their high level of power performance, SCs are still too expensive to compete against the other available electrochemical energy storage systems, typically batteries. It should also be noted that lowering the cost without sacrificing the long cycle life and the exceptional high rate performance which distinguish ECs from batteries, is essential.In this review, we have summarized recent advancements of nanoarray electrodes for SCs. As a new class of electrode materials, nanoarray electrodes can endow SCs with their unique properties (such as fast electron transport, superior ion diffusion, high electrical conductivity and excellent structural mechanical stability) providing a promising avenue to boost the electrochemical performance of nanomaterials. Accordingly, the nanoarray electrode-based SCs show promising potential in achieving both high energy density and high power density. Further work on nanoarray electrodes is expected to focus on the following areas: first, to develop controllable and cost-effective preparation protocols which should also be easy to scale up. Second, to understand how the spatial structure of nanoarrays contributes to different aspects including the electronic transport, interfacial properties and electrochemical performances. Thirdly, to develop nanoarrays on flexible substrates for special applications.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant no. 51302079). We also thank Dr Tania Silver from Institute for Superconducting and Electronic Materials (University of Wollongong) for revising our manuscript.Notes and references
- J. P. Holdren, Science, 2007, 315, 737 CrossRef CAS PubMed.
- M. Winter and R. J. Brodd, Chem. Rev., 2004, 104, 4245 CrossRef CAS.
- Z. Yang, J. Zhang, M. C. W. Kintner-Meyer, X. Lu, D. Choi, J. P. Lemmon and J. Liu, Chem. Rev., 2011, 111, 3577 CrossRef CAS PubMed.
- C. Largeot, C. Portet, J. Chmiola, P. Taberna, Y. Gogotsi and P. Simon, J. Am. Chem. Soc., 2008, 130, 2730 CrossRef CAS PubMed.
- S. Kandalkar, D. Dhawale, C. Kim and C. Lokhande, Synth. Met., 2010, 160, 1299 CrossRef CAS PubMed.
- M. D. Stoller and R. S. Ruoff, Energy Environ. Sci., 2010, 3, 1294 CAS.
- P. J. Hall, M. Mirzaeian, S. I. Fletcher, F. B. Sillars, A. J. R. Rennie, G. O. Shitta-Bey, G. Wilson, A. Cruden and R. Carter, Energy Environ. Sci., 2010, 3, 1238 CAS.
- L. L. Zhang and X. S. Zhao, Chem. Soc. Rev., 2009, 38, 2520 RSC.
- H. Jiang, L. P. Yang, C. Z. Li, C. Y. Yan, P. S. Lee and J. Ma, Energy Environ. Sci., 2011, 4, 1813 CAS.
- B. E. Conway, Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications, KluwerAcademic/Plenum Publisher, New York, 1999 Search PubMed.
- H. Jiang, J. Ma and C. Z. Li, Adv. Mater., 2012, 24, 4197 CrossRef CAS PubMed.
- G. H. Yu, X. Xie, L. J. Pan, Z. N. Bao and Y. Cui, Nano Energy, 2013, 2, 213 CrossRef CAS PubMed.
- X. Y. Chen, E. Pomerantseva, P. Banerjee, K. Gregorczyk, R. Ghodssi and G. Rubloff, Chem. Mater., 2012, 24, 1255 CrossRef CAS.
- J. S. Shaikh, R. C. Pawar, R. S. Devan, Y. R. Ma, P. P. Salvi, S. S. Kolekar and P. S. Patil, Electrochim. Acta, 2011, 56, 2127 CrossRef CAS PubMed.
- M. J. Zhi, C. C. Xiang, J. T. Li, M. Li and N. Q. Wu, Nanoscale, 2013, 5, 72 RSC.
- V. D. Patake, C. D. Lokhande and O. S. Joo, Appl. Surf. Sci., 2009, 255, 4192 CrossRef CAS PubMed.
- U. M. Patil, R. R. Salunkhe, K. V. Gurav and C. D. Lokhande, Appl. Surf. Sci., 2008, 255, 2603 CrossRef CAS PubMed.
- B. R. Duan and Q. Cao, Electrochim. Acta, 2012, 64, 154 CrossRef CAS PubMed.
- R. Z. Li, X. Ren, F. Zhang, C. Du and J. P. Liu, Chem. Commun., 2012, 48, 5010 RSC.
- G. L. Wang, J. C. Huang, S. L. Chen, Y. Y. Gao and D. X. Cao, J. Power Sources, 2011, 196, 5756 CrossRef CAS PubMed.
- S. K. Mondal and N. Munichandraiah, J. Power Sources, 2008, 175, 657 CrossRef CAS PubMed.
- X. H. Lu, G. M. Wang, T. Zhai, M. H. Yu, J. Y. Gan, Y. X. Tong and Y. Li, Nano Lett., 2012, 12, 1690 CrossRef CAS PubMed.
- X. H. Lu, G. M. Wang, T. Zhai, M. H. Yu, S. L. Xie, Y. C. Ling, C. L. Liang, Y. X. Tong and Y. Li, Nano Lett., 2012, 12, 5376 CrossRef CAS PubMed.
- R. Liu and S. B. Lee, J. Am. Chem. Soc., 2008, 130, 2942 CrossRef CAS PubMed.
- K. Naoi and M. Morita, Electrochem. Soc. Interface, 2008, 17, 44 CAS.
- J. Jang, J. Bae, M. Choi and S. H. Yoon, Carbon, 2005, 43, 2730 CrossRef CAS PubMed.
- X. Yan, Z. Tai, J. Chen and Q. Xue, Nanoscale, 2011, 3, 212 RSC.
- L. Pan, H. Qiu, C. Dou, Y. Li, L. Pu, J. Xu and Y. Shi, Int. J. Mol. Sci., 2010, 11, 2636 CrossRef CAS PubMed.
- Y. Horng, Y. C. Lu, Y. K. Hsu, C. C. Chen, L. C. Chen and K. H. Chen, J. Power Sources, 2010, 195, 4418 CrossRef CAS PubMed.
- C. Z. Yuan, B. Gao, L. F. Shen, S. D. Yang, L. Hao, X. J. Lu, F. Zhang, L. J. Zhang and X. G. Zhang, Nanoscale, 2011, 3, 529 RSC.
- J. Jiang, Y. Y. Li, J. P. Liu, X. T. Huang, C. Z. Yuan and X. W. Lou, Adv. Mater., 2012, 24, 5166 CrossRef CAS PubMed.
- Y. C. Liu and K. Y. Xie, Sci. Adv. Mater., 2014, 6, 863 CrossRef CAS PubMed.
- B. Kang and G. Ceder, Nature, 2009, 458, 190 CrossRef CAS PubMed.
- H. S. Zhou, D. L. Li, M. Hibino and I. Honma, Angew. Chem., Int. Ed., 2005, 44, 797–802 CrossRef CAS PubMed.
- K. M. Shaju and P. G. Bruce, Adv. Mater., 2006, 18, 2330 CrossRef CAS PubMed.
- K. S. Kang, Y. S. Meng, J. Breger, C. P. Grey and G. Ceder, Science, 2006, 311, 977 CrossRef CAS PubMed.
- C. Liu, F. Li, L. P. Ma and H. M. Cheng, Adv. Mater., 2010, 22, E28 CrossRef CAS PubMed.
- L. J. Fu, H. Liu, C. Li, Y. P. Wu, E. Rahm, R. Holze and H. Q. Wu, Solid State Sci., 2006, 8, 113 CrossRef CAS PubMed.
- Y. G. Wang, Y. R. Wang, E. J. Hosono, K. X. Wang and H. S. Zhou, Angew. Chem., Int. Ed., 2008, 47, 7461 CrossRef CAS PubMed.
- C. K. Chan, H. L. Peng, G. Liu, K. McIlwrath, X. F. Zhang, R. A. Huggins and Y. Cui, Nat. Nanotechnol., 2008, 3, 31 CrossRef CAS PubMed.
- N. C. Li, C. J. Patrissi, G. L. Che and C. R. Martin, J. Electrochem. Soc., 2000, 147, 2044 CrossRef CAS PubMed.
- L. Taberna, S. Mitra, P. Poizot, P. Simon and J. M. Tarascon, Nat. Mater., 2006, 5, 567 CrossRef PubMed.
- T. Fang, J. G. Duh and S. R. Sheen, J. Electrochem. Soc., 2005, 152, A1701 CrossRef CAS PubMed.
- H. S. Zhou, D. L. Li, M. Hibino and I. Honma, Angew. Chem., Int. Ed., 2005, 44, 797 CrossRef CAS PubMed.
- L. W. Ji, Z. Lin, M. Alcoutlabi and X. W. Zhang, Energy Environ. Sci., 2011, 4, 2682 CAS.
- F. Y. Cheng, J. Liang, Z. L. Tao and J. Chen, Adv. Mater., 2011, 23, 1695 CrossRef CAS PubMed.
- H. Li, Z. X. Wang, L. Q. Chen and X. J. Huang, Adv. Mater., 2009, 21, 4593 CrossRef CAS PubMed.
- Y. G. Wang, H. Q. Li, P. He, E. Hosono and H. S. Zhou, Nanoscale, 2010, 2, 1294 RSC.
- Z. Chen, V. Augustyn, J. Wen, Y. W. Zhang, M. Q. Shen, B. Dunn and Y. F. Lu, Adv. Mater., 2011, 23, 791 CrossRef CAS PubMed.
- G. Xiong, C. Meng, R. G. Reifenberger, P. P. Irazoqui and T. S. Fisher, Electroanalysis, 2014, 26(1), 30 CrossRef CAS PubMed.
- M. Beidaghi and Y. Gogotsi, Energy Environ. Sci., 2014, 7, 867 CAS.
- H. Jiang, P. S. Lee and C. Z. Li, Energy Environ. Sci., 2013, 6, 41 CAS.
- C. Zhou, Y. W. Zhang, Y. Y. Li and J. P. Liu, Nano Lett., 2013, 13, 2078 CrossRef CAS PubMed.
- G. H. Zhang, T. H. Wang, X. Z. Yu, H. N. Zhang, H. G. Duan and B. A. Lu, Nano Energy, 2013, 2, 586 CrossRef CAS PubMed.
- D. Ghosh, S. Giri and C. K. Das, Nanoscale, 2013, 5, 10428 RSC.
- J. P. Liu, J. Jiang, C. W. Cheng, H. X. Li, J. X. Zhang, H. Gong and H. J. Fan, Adv. Mater., 2011, 23, 2076 CrossRef CAS PubMed.
- M. C. Liu, L. B. Kong, C. Lu, X. J. Ma, X. M. Li, Y. C. Luo and L. Kang, J. Mater. Chem. A, 2013, 1, 1380 CAS.
- X. Y. Liu, S. J. Shi, Q. Q. Xiong, L. Li, Y. J. Zhang, H. Tang, C. D. Gu, X. L. Wang and J. P. Tu, ACS Appl. Mater. Interfaces, 2013, 5, 8790 CAS.
- Y. S. Luo, J. Jiang, W. W. Zhou, H. P. Yang, J. S. Luo, X. Y. Qi, H. Zhang, D. Y. W. Yu, C. M. Li and T. Yu, J. Mater. Chem., 2012, 22, 8634 RSC.
- H. Jiang, J. Ma and C. Z. Li, J. Mater. Chem., 2012, 22, 16939 RSC.
- X. H. Xia, J. P. Tu, Y. Q. Zhang, X. L. Wang, C. D. Gu, X. B. Zhao and H. J. Fan, ACS Nano, 2012, 6, 5531 CrossRef CAS PubMed.
- G. P. Wang, L. Zhang and J. J. Zhang, Chem. Soc. Rev., 2012, 41, 797 RSC.
- H. M. Zhang, Y. J. Chen, W. W. Wang, G. H. Zhang, M. Zhuo, H. M. Zhang, T. Yang, Q. H. Li and T. H. Wang, J. Mater. Chem. A, 2013, 1, 8593 CAS.
- L. Yu, G. Q. Zhang, C. Z. Yuan and X. W. Lou, Chem. Commun., 2013, 49, 137 RSC.
- R. L. Liang, H. Q. Cao and D. Qian, Chem. Commun., 2011, 47, 10305 RSC.
- C. Guan, J. P. Liu, C. W. Cheng, H. X. Li, X. L. Li, W. W. Zhou, H. Zhang and H. J. Fan, Energy Environ. Sci., 2011, 4, 4496 CAS.
- L. L. Hu, B. H. Qu, C. C. Li, Y. J. Chen, L. Mei, D. N. Lei, L. B. Chen, Q. H. Li and T. H. Wang, J. Mater. Chem. A, 2013, 1, 5596 CAS.
- X. Xiao, T. P. Ding, L. Y. Yuan, Y. Q. Shen, Q. Z. Zhong, X. H. Zhang, Y. Z. Cao, B. Hu, T. Zhai, L. Gong, J. Chen, Y. X. Tong, J. Zhou and Z. L. Wang, Adv. Energy Mater., 2012, 2, 1328 CrossRef CAS PubMed.
- D. Guo, H. M. Zhang, X. Z. Yu, M. Zhang, P. Zhang, Q. H. Li and T. H. Wang, J. Mater. Chem. A, 2013, 1, 7247 CAS.
- L. Q. Mai, F. Yang, Y. L. Zhao, X. Xu, L. Xu and Y. Z. Luo, Nat. Commun., 2011, 2, 381 CrossRef PubMed.
- D. Guo, P. Zhang, H. M. Zhang, X. Z. Yu, J. Zhu, Q. H. Li and T. H. Wang, J. Mater. Chem. A, 2013, 1, 9024 CAS.
- H. Y. Lee and J. B. Goodenough, J. Solid State Chem., 1999, 144, 220 CrossRef CAS.
- A. G. Pandolfo and A. F. Hollenkamp, J. Power Sources, 2006, 157, 11 CrossRef CAS PubMed.
- D. S. Su and R. Schlogl, ChemSusChem, 2010, 3, 136 CrossRef CAS PubMed.
- B. Kim, H. Chung and W. Kim, J. Phys. Chem. C, 2010, 114, 15225 Search PubMed.
- G. L. Che, B. B. Lakshmi, E. R. Fisher and C. R. Martin, Nature, 1998, 393, 346 CrossRef CAS.
- W. Y. Li, L. N. Xu and J. Chen, Adv. Funct. Mater., 2005, 15, 851 CrossRef CAS PubMed.
- X. X. Li, F. Y. Cheng, B. Guo and J. Chen, J. Phys. Chem. B, 2005, 109, 14017 CrossRef CAS PubMed.
- M. G. Hahm, A. L. M. Reddy, D. P. Cole, M. Rivera, J. A. Vento, J. Nam, H. Y. Jung, Y. L. Kim, N. T. Narayanan, D. P. Hashim, C. Galande, Y. J. Jung, M. Bundy, S. Karna, P. M. Ajayan and R. Vajtai, Nano Lett., 2012, 12, 5616 CrossRef CAS PubMed.
- A. Rudge, J. Davey, I. Raistrick, S. Gottesfeld and J. Ferraris, J. Power Sources, 1994, 47, 89 CrossRef CAS.
- D. K. Bhat and M. S. Kumar, J. Mater. Sci., 2007, 42, 8158 CrossRef CAS PubMed.
- C. X. Guo, M. Wang, T. Chen, X. W. Lou and C. M. Li, Adv. Energy Mater., 2011, 1, 736 CrossRef CAS PubMed.
- K. Wang, J. Y. Huang and Z. X. Wei, J. Phys. Chem. C, 2010, 114, 8062 CAS.
- K. Wang, W. J. Zou, B. G. Quan, A. F. Yu, H. P. Wu, P. Jiang and Z. X. Wei, Adv. Energy Mater., 2011, 1(6), 1068 CrossRef CAS PubMed.
- J. Y. Huang, K. Wang and Z. X. Wei, J. Mater. Chem., 2010, 20, 1117 RSC.
- J. Liu, G. Z. Cao, Z. G. Yang, D. H. Wang, D. Dubois, X. D. Zhou, G. L. Graff, L. R. Pederson and J. G. Zhang, ChemSusChem, 2008, 1, 676 CrossRef CAS PubMed.
- C. Z. Yuan, L. Yang, L. R. Hou, L. F. Shen, X. G. Zhang and X. W. Lou, Energy Environ. Sci., 2012, 5, 7883 CAS.
- C. Z. Yuan, J. Y. Li, L. R. Hou, X. G. Zhang, L. F. Shen and X. W. Lou, Adv. Funct. Mater., 2012, 22, 4592 CrossRef CAS PubMed.
- J. T. Li, W. Zhao, F. Q. Huang, A. Manivannan and N. Q. Wu, Nanoscale, 2011, 3, 5103 RSC.
- Y. Y. Gao, S. L. Chen, D. X. Cao, G. L. Wang and J. L. Yin, J. Power Sources, 2010, 195, 1757 CrossRef CAS PubMed.
- G. Q. Zhang, H. B. Wu, H. E. Hoster, M. B. Chan-Park and X. W. Lou, Energy Environ. Sci., 2012, 5, 9453 CAS.
- G. Q. Zhang and X. W. Lou, Adv. Mater., 2013, 25, 976 CrossRef CAS PubMed.
- L. H. Bao, J. F. Zang and X. D. Li, Nano Lett., 2011, 11, 1215 CrossRef CAS PubMed.
- B. Liu, B. Y. Liu, Q. F. Wang, X. F. Wang, Q. Y. Xiang, D. Chen and G. Z. Shen, ACS Appl. Mater. Interfaces, 2013, 5, 10011 CAS.
- J. W. Liu, J. Essner and J. Li, Chem. Mater., 2010, 22, 5022 CrossRef CAS.
- J. P. Liu, C. W. Cheng, W. W. Zhou, H. X. Li and H. J. Fan, Chem. Commun., 2011, 47, 3436 RSC.
- K. S. Park, J. G. Kang, Y. J. Choi, S. J. Lee, D. W. Kim and J. G. Park, Energy Environ. Sci., 2011, 4, 1796 CAS.
- Y. C. Liu and K. Y. Xie, Sci. Adv. Mater., 2014, 6, 1947 Search PubMed.
- H. Zhang, G. Cao, Z. Wang, Y. Yang, Z. Shi and Z. Gu, Nano Lett., 2008, 8, 2664 CrossRef CAS PubMed.
- X. W. Cui, F. P. Hu, W. F. Wei and W. X. Chen, Carbon, 2011, 49, 1225 CrossRef CAS PubMed.
- X. Z. Yu, B. A. Lu and Z. Xu, Adv. Mater., 2014, 26, 1044 CrossRef CAS PubMed.
- Z. L. Wang, R. Guo, L. X. Ding, Y. X. Tong and G. R. Li, Sci. Rep., 2013, 3, 1204 Search PubMed.
- H. Zhang, G. Cao, Z. Wang, Y. Yang, Z. Shi and Z. Gu, Electrochem. Commun., 2008, 10, 1056 CrossRef CAS PubMed.
- K. Wang, Q. H. Meng, Y. J. Zhang, Z. X. Wei and M. H. Miao, Adv. Mater., 2013, 25, 1494 CrossRef CAS PubMed.
- J. J. Xu, K. Wang, S. Z. Zu, B. H. Han and Z. X. Wei, ACS Nano, 2010, 4, 5019 CrossRef CAS PubMed.
- X. H. Xia, J. P. Tu, Y. Q. Zhang, J. Chen, X. L. Wang, C. D. Gu, C. Guan, J. S. Luo and H. J. Fan, Chem. Mater., 2012, 24, 3793 CrossRef CAS.
- W. Tian, X. Wang, C. Y. Zhi, T. Y. Zhai, D. Q. Liu, C. Zhang, D. Golberg and Y. Bando, Nano energy, 2013, 2, 754 CrossRef CAS PubMed.
- F. F. Cao, J. W. Deng, S. Xin, H. X. Ji, O. G. Schmidt, L. J. Wan and Y. G. Guo, Adv. Mater., 2011, 23, 4415 CrossRef CAS PubMed.
- Y. Qi, N. Du, H. Zhang, X. Fan, Y. Yang and D. R. Yang, Nanoscale, 2012, 4, 991 RSC.
- J. W. Long, B. Dunn, D. R. Rolison and H. S. White, Chem. Rev., 2004, 104, 4463 CrossRef CAS.
- J. Hassoun, S. Panero, P. Simon, P. L. Taberna and B. Scrosati, Adv. Mater., 2007, 19, 1632 CrossRef CAS PubMed.
- F. Lu, M. Qiu, X. Qi, L. Yang, J. Yi, G. Hao, X. Feng, J. Li and J. Zhong, Appl. Phys. A: Mater. Sci. Process., 2011, 104, 545 CrossRef CAS PubMed.
- Y. Sun, Q. Wu and G. Shi, Energy Environ. Sci., 2011, 4, 1113 CAS.
- W. Gao, N. Singh, L. Song, Z. Liu, A. L. M. Reddy, L. Ci, R. Vajtai, Q. Zhang, B. Wei and P. M. Ajayan, Nat. Nanotechnol., 2011, 6, 496 CrossRef CAS PubMed.
- J. R. Miller, R. A. Outlaw and B. C. Holloway, Science, 2010, 329, 1637 CrossRef CAS PubMed.
- R. Liu, J. Duay and S. B. Lee, Chem. Commun., 2011, 47, 1384 RSC.
- D. R. Rolison, J. W. Long, J. C. Lytle, A. E. Fischer, C. P. Rhodes, T. M. McEvoy, M. E. Bourg and A. M. Lubers, Chem. Soc. Rev., 2009, 38, 226 RSC.
- Z. Chen, W. Ren, L. Gao, B. Liu, S. Pei and H. M. Cheng, Nat. Mater., 2011, 10, 424 CrossRef CAS PubMed.
- W. Chen, S. Li, C. Chen and L. Yan, Adv. Mater., 2011, 23, 5679 CrossRef CAS PubMed.
- Y. Wang, C. X. Guo, X. Wang, C. Guan, H. Yang, K. Wang and C. M. Li, Energy Environ. Sci., 2011, 4, 195 CAS.
- T. Maiyalagan, X. Dong, P. Chen and X. Wang, J. Mater. Chem., 2012, 22, 5286 RSC.
- X. C. Dong, H. Xu, X. W. Wang, Y. X. Huang, M. B. Chan-Park, H. Zhang, L. H. Wang, W. Huang and P. Chen, ACS Nano, 2012, 6, 3206 CrossRef CAS PubMed.
- Y. He, W. Chen, X. Li, Z. Zhang, J. Fu, C. Zhao and E. Xie, ACS Nano, 2012, 7, 174 CrossRef PubMed.
- Z. J. Fan, J. Yan, L. J. Zhi, Q. Zhang, T. Wei, J. Feng, M. L. Zhang, W. Z. Qian and F. Wei, Adv. Mater., 2010, 22, 3723 CrossRef CAS PubMed.
- M. Wang, Q. Liu, H. Sun, E. A. Stach, H. Zhang, L. Stanciu and J. Xie, Carbon, 2012, 50, 3845 CrossRef CAS PubMed.
- X. W. Yang, J. W. Zhu, L. Qiu and D. Li, Adv. Mater., 2011, 23, 2833 CrossRef CAS PubMed.
- S. Zhang, Y. Li and N. Pan, J. Power Sources, 2012, 206, 476 CrossRef CAS PubMed.
- J. Chen, K. Sheng, P. Luo, C. Li and G. Shi, Adv. Mater., 2012, 24, 4569 CrossRef CAS PubMed.
- B. G. Choi, M. H. Yang, W. H. Hong, J. W. Choi and Y. S. Huh, ACS Nano, 2012, 6, 4020 CrossRef CAS PubMed.
- X. H. Cao, Y. M. Shi, W. H. Shi, G. Lu, X. Huang, Q. Y. Yan, Q. C. Zhang and H. Zhang, Small, 2011, 7, 3163 CrossRef CAS PubMed.
- S. Hu, R. Rajamani and X. Yu, Appl. Phys. Lett., 2012, 100, 104103 CrossRef PubMed.
- H. Nishide and K. Oyaizu, Science, 2008, 319, 737 CrossRef CAS PubMed.
- M. Kaempgen, C. K. Chan, J. Ma, Y. Cui and G. Gruner, Nano Lett., 2009, 9, 1872 CrossRef CAS PubMed.
- Y. Yang, G. D. Ruan, C. S. Xiang, G. Wang and J. M. Tour, J. Am. Chem. Soc., 2014, 136, 6187 CrossRef CAS PubMed.
- L. B. Hu, M. Pasta, F. L. Mantia, L. F. Cui, S. M. Jeong, H. D. Deshazer, J. W. Choi, S. M. Han and Y. Cui, Nano Lett., 2010, 10, 708 CrossRef CAS PubMed.
- L. B. Hu, H. Wu, F. L. Mantia, Y. Yang and Y. Cui, ACS Nano, 2010, 4, 5843 CrossRef CAS PubMed.
- M. Pasta, F. L. Mantia, L. B. Hu, H. D. Deshazer and Y. Cui, Nano Res., 2010, 3, 452 CrossRef CAS PubMed.
- G. Yu, L. B. Hu, M. Vosgueritchian, H. L. Wang, X. Xie, J. R. McDonough, X. Cui, Y. Cui and Z. N. Bao, Nano Lett., 2011, 11, 2905 CrossRef CAS PubMed.
- C. Z. Meng, C. H. Liu, L. Z. Chen, C. H. Hu and S. S. Fan, Nano Lett., 2010, 10, 4025 CrossRef CAS PubMed.
- L. H. Bao and X. D. Li, Adv. Mater., 2012, 24, 3246 CrossRef CAS PubMed.
- X. H. Lu, T. Zhai, X. H. Zhang, Y. Q. Shen, L. Y. Yuan, B. Hu, L. Gong, J. Chen, Y. H. Gao, J. Zhou, Y. X. Tong and Z. L. Wang, Adv. Mater., 2012, 24, 938 CrossRef CAS PubMed.
- Y. C. Chen, Y. K. Hsu, Y. G. Lin, Y. K. Lin, Y. Y. Horng, L. C. Chen and K. H. Chen, Electrochim. Acta, 2011, 56, 7124 CrossRef CAS PubMed.
- L. Y. Yuan, X. H. Lu, X. Xiao, T. Zhai, J. J. Dai, F. C. Zhang, B. Hu, X. Wang, L. Gong, J. Chen, C. G. Hu, Y. X. Tong, J. Zhou and Z. L. Wang, ACS Nano, 2012, 6, 656 CrossRef CAS PubMed.
- Y. K. Hsu, Y. C. Chen, Y. G. Lin, L. C. Chen and K. H. Chen, J. Mater. Chem., 2012, 22, 3383 RSC.
- M. H. Yu, T. Zhai, X. H. Lu, X. J. Chen, S. L. Xie, W. Li, C. L. Liang, W. X. Zhao, L. P. Zhang and Y. X. Tong, J. Power Sources, 2013, 239, 64 CrossRef CAS PubMed.
- J. Xu, Q. F. Wang, X. W. Wang, Q. Y. Xiang, B. Liang, D. Chen and G. Z. Shen, ACS Nano, 2013, 7, 5453 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |
