Mechanical properties and degradation studies of poly(mannitol sebacate)/cellulose nanocrystals nanocomposites†
Águeda Sonseca*a,
Oscar Sahuquilloa,
E. Johan Fosterb and
Enrique Giméneza
aInstituto de Tecnología de Materiales, Universitat Politècnica de València (UPV), Camino de Vera s/n, 46022 Valencia, Spain. E-mail: agsonol@posgrado.upv.es; Tel: +34 963879625
bVirginia Tech, Department of Materials Science & Engineering, 445 Old Turner Street, 213 Holden Hall, Blacksburg, VA 24061, USA
First published on 19th June 2015
Abstract
Polyesters based on polyols and sebacic acid, known as poly(polyol sebacate)s (PPS) are good candidates to develop degradable materials, due to their combination of flexibility and degradability, which are both useful properties in the context of soft-tissue engineering (Z. Sun, C. Chen, M. Sun, C. Ai, X. Lu, Y. Zheng, B. Yang and D. Dong, Biomaterials, 2009, 30, 5209, C. Sundback, J. Shyu, Y. Wang, W. Faquin, R. Langer, J. Vacanti and T. Hadlock, Biomaterials, 2005, 26, 5454, D. Motlagh, J. Yang, K. Lui, A. Webb and G. Ameer, Biomaterials, 2006, 27, 4315, A. Mahdavi, L. Ferreira, C. Sundback, J. W. Nichol, E. P. Chan, D. J. D. Carter, C. J. Bettinger, S. Patanavanich, L. Chignozha, E. Ben-Joseph, A. Galakatos, H. Pryor, I. Pomerantseva, P. T. Masiakos, W. Faquin, A. Zumbuehl, S. Hong, J. Borenstein, J. Vacanti, R. Langer and J. M. Karp, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 2307). However, PPS generally display poor mechanical properties, in particular a low modulus, that limit the true potential of these materials in the biomedical field. Here, we introduce an approach to obtain nanocomposites based on poly(mannitol sebacate) (PMS) matrices reinforced with cellulose nanocrystals (CNCs) in order to improve the application range of these materials. Different strategies were used based on varying the feed ratios between mannitol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sebacic acid (1
sebacic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), crosslinking conditions and CNCs content, resulting in different degrees of crosslinking and, therefore, mechanical and degradation behavior. All of the developed nanocomposites displayed the expected mass loss during the degradation studies in simulated body fluid (SBF) similar to the neat matrix, however, doubling the sebacic acid feed ratio or extending the curing temperature and time, resulted in higher mechanical properties, structural integrity, and shape stability during a degradation time lessening mass loss rate. Changing mannitol
2), crosslinking conditions and CNCs content, resulting in different degrees of crosslinking and, therefore, mechanical and degradation behavior. All of the developed nanocomposites displayed the expected mass loss during the degradation studies in simulated body fluid (SBF) similar to the neat matrix, however, doubling the sebacic acid feed ratio or extending the curing temperature and time, resulted in higher mechanical properties, structural integrity, and shape stability during a degradation time lessening mass loss rate. Changing mannitol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sebacic acid reaction ratios from 1
sebacic acid reaction ratios from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and for low crosslinking degree neat samples, the Young's modulus increases four-fold, while mass loss after 150 days of incubation is reduced by half. The Young's modulus range obtained with this process covers the range of human elastic soft tissues to tough tissues (0.7–200 MPa).
2 and for low crosslinking degree neat samples, the Young's modulus increases four-fold, while mass loss after 150 days of incubation is reduced by half. The Young's modulus range obtained with this process covers the range of human elastic soft tissues to tough tissues (0.7–200 MPa).
Introduction
In the past few years, there has been steady progress in the development of biodegradable polymers for application in the medical field because these materials provide new opportunities to design less invasive and resorbable implants, tissue scaffolds, and medical devices that are able to avoid second revision surgeries, that limit the risk of infection and results in less trauma for the patient.5–8 Moreover, biodegradable polymeric systems which offer the possibility to tailor their mechanical properties from soft to stiffer as well as the biodegradability ratio by varying the processing conditions also have additional advantages. Medical devices based on different classes of biodegradable polymers such as polyesters, polyanhydrides and polyurethanes have been widely investigated and some of them are commercially available as resorbable sutures, drug delivery systems, vascular grafts, wafers and orthopaedic fixation devices based, among others, on polylactic acid (PLA), polyglycolic acid (PGA), poly(lactic-co-glycolic acid) (PLGA), poly(ε-caprolactone) (PCL), poly(p-dioxanone) (PDO), or poly(trimethylene carbonate) (PTMC).9,10 However, most of these polymeric systems have degradation rates that are not optimal for short-medium term applications. For example, it takes more than 24 months for poly(caprolactones) and poly(L-lactic acid) to degrade in a biological environment, and from 2 to 24 months, depending on the ratio, for copolymers of polylactic acid (PLA) and polyglycolic acid (PGA). Also, the degradation rate for polyanhydrides is slow, around 12 months.6,9,11 Moreover, these polymers have a lack of flexibility and elasticity at body temperature due to their high glass transition temperature and too high elastic modulus (400 MPa for PCL and 1200–3000 MPa for PLA)12 that fail to mimic the elastic nature of many soft tissues such as blood vessels, cartilage, tendons or ligaments causing irritation, and sometimes damage, to the surrounding tissues.13–15 To overcome these shortcomings, the development of novel biodegradable polyester elastomers, which are able to control their elastic properties and flexibility to better match with the elastic nature, biocompatibility and degradation profile of soft tissues, has increased considerably.16,17 Several researchers have synthesized polyester elastomers based on polyols such as 1,8-octanediol, ethylene glycol, butylene glycol, castor oil or glycerol, and carboxylic acids such as citric acid, ricinoleic acid, and sebacic acid,18–25 which find application in soft-tissue engineering as nerve-guidance, drug delivery, tissue adhesives and scaffolds to repair or replace body tissues.1–4,19,26,27 Poly(glycerol sebacate) (PGS) represents the most studied member of the poly-polyol sebacate (PPS) family, all of which are attractive because they are inexpensive and endogenous monomers found in human metabolism.27–29 PGS is a randomly crosslinked polyester elastomer, whose design was motivated by the need to find biodegradable materials with tough mechanical properties.26 Since PGS was firstly reported, several other polyols have been polymerized with sebacic acid to cover a wide range of mechanical properties and expand the spectrum of biomedical applications for these materials.16,27,30 The most attractive synthetic feature of PPS is that through altering the stoichiometry of the diacid and polyol as well as varying the polycondensation conditions, the mechanical properties and degradation rates can be easily tuned. Moreover, PPS have biodegradability under physiological conditions and acceptable biocompatibility comparable to PLGA,16,31 and they have been reported as non-toxic based on in vitro32 and in vivo studies.33 These results indicate that these materials will be successful biomaterials with many biomedical applications. These thermoset elastomers have been reported to degrade via hydrolysis by surface erosion into metabolizable products, that maintain their structural integrity and geometric stability, and they are able to provide mechanical support during in vivo degradation.30,33,34 The mechanical properties of PPS (tensile strength and elastic modulus) can be enhanced within a modest range by modifying the crosslink densities or in a much wider range by adjusting the stoichiometry. Nevertheless, to ensure an elongation at break higher than 10% (required for most tissue engineering applications), the range of Young's modulus for these materials (0.05–13 MPa) does not entirely cover that of living tissues such as skin (0.7–16 MPa), ligaments (1.5–54.5 MPa), tendons (1.5 GPa), or mandibular trabecular bone (6.9–200 MPa).14,35,36 This fact restricts their usefulness in applications where high strength and flexibility are required, such as ligating rubber bands for blood vessels or bones, elastomeric sutures, flexible coatings for stents, surgical devices, vascular grafts, surgical wound dressings, or catheters and small diameter tubes for drainage.37 In this regard, we have reported in a previous work38 that the addition of cellulose nanocrystals (CNCs) as a reinforcing filler in a poly(mannitol sebacate) (PMS) matrix with mannitol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sebacic acid ratio 1
sebacic acid ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, is an effective approach to increase the strength and stiffness without compromising the elongation at break due to a strong affinity between matrix and filler, and the superior specific properties of the CNC (specific Young's modulus ∼ 100 GPa cm−3 g−1)39,40 compared with other nanofillers such as clays or bioceramics (Bioglass®). In that work, we developed 1
1, is an effective approach to increase the strength and stiffness without compromising the elongation at break due to a strong affinity between matrix and filler, and the superior specific properties of the CNC (specific Young's modulus ∼ 100 GPa cm−3 g−1)39,40 compared with other nanofillers such as clays or bioceramics (Bioglass®). In that work, we developed 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PMS nanocomposites with 1, 5 and 10 wt% of CNCs using the solution casting method followed by two different thermal crosslinking profiles, under low and high temperature-time conditions, to achieve samples with high and low degree of crosslinking. Mechanical, thermal, thermomechanical, and shape-memory properties of the samples were then evaluated, and the results indicated that at least in a certain extent CNCs interact with the matrix either, physically and chemically.41,42 However, as was mentioned above, different ratio of mannitol and sebacic acid in PMS will hardly influence the properties of the matrix as well as the interactions with the filler. Therefore, to complete our previous study,38 the aim of the present work is to develop PMS/CNC nanocomposites with mannitol
1 PMS nanocomposites with 1, 5 and 10 wt% of CNCs using the solution casting method followed by two different thermal crosslinking profiles, under low and high temperature-time conditions, to achieve samples with high and low degree of crosslinking. Mechanical, thermal, thermomechanical, and shape-memory properties of the samples were then evaluated, and the results indicated that at least in a certain extent CNCs interact with the matrix either, physically and chemically.41,42 However, as was mentioned above, different ratio of mannitol and sebacic acid in PMS will hardly influence the properties of the matrix as well as the interactions with the filler. Therefore, to complete our previous study,38 the aim of the present work is to develop PMS/CNC nanocomposites with mannitol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sebacic acid 1
sebacic acid 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometry, using the same CNCs loading and crosslinking profiles than for previously reported 1
2 stoichiometry, using the same CNCs loading and crosslinking profiles than for previously reported 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PMS stoichiometry, to compare how mechanical, thermal and degradation properties are affected. For PMS matrix with mannitol
1 PMS stoichiometry, to compare how mechanical, thermal and degradation properties are affected. For PMS matrix with mannitol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sebacic acid 1
sebacic acid 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometry, we have also demonstrated that the residual alcohol groups of PMS matrix serve as ideal scaffolds for covalent tethering of chromophores with potential applications as bio-based up-converting rubbery systems for drug-delivery, bio-imaging, solar harvesting or displays.43 This previous experience demonstrates the possibility to covalently attach chromophores to a PMS matrix. In this regard, PMS/CNC nanocomposites with 1
2 stoichiometry, we have also demonstrated that the residual alcohol groups of PMS matrix serve as ideal scaffolds for covalent tethering of chromophores with potential applications as bio-based up-converting rubbery systems for drug-delivery, bio-imaging, solar harvesting or displays.43 This previous experience demonstrates the possibility to covalently attach chromophores to a PMS matrix. In this regard, PMS/CNC nanocomposites with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio were developed and final properties were tuned and evaluated taking into account the possibility to be influenced either by physical and chemical CNC/PMS interactions, but in a different way than for 1
2 ratio were developed and final properties were tuned and evaluated taking into account the possibility to be influenced either by physical and chemical CNC/PMS interactions, but in a different way than for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PMS system, due to the stark differences of the matrices. Here we reported an approach build upon previous work, showing a study of PMS/CNC nanocomposites from mannitol
1 PMS system, due to the stark differences of the matrices. Here we reported an approach build upon previous work, showing a study of PMS/CNC nanocomposites from mannitol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sebacic acid 1
sebacic acid 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 reaction ratios with low and high crosslinking degrees that were able to cover a wide range of mechanical properties and degradation rates for potential biomedical applications.
2 reaction ratios with low and high crosslinking degrees that were able to cover a wide range of mechanical properties and degradation rates for potential biomedical applications.
Experimental section
Materials
Deuterated dimethylsulfoxide (DMSO-d6), dimethylformamide (DMF), sulfuric acid, sebacic acid (SA) (99% purity), and D-mannitol (MA) (99% purity), were purchased from Sigma Aldrich. Hydrochloric acid (HCl, 37% reagent grade) and sodium hydroxide (NaOH, 98% reagent grade) were purchased from Scharlau. Ultrapure water was directly taken from a Sartorius Stedim Arium 611 VF® water purification system (ρ = 18.2 mΩ cm). Cellulose nanocrystals were isolated from cotton (Whatman no. 1 filter paper) by controlled hydrolysis with sulphuric acid according to a protocol that is a modification of the method originally described by Dong et al.44 This protocol introduces a small concentration of sulfate ester groups on the surface of the CNCs, which help to form stable suspensions in polar solvents.45 Protocols followed to analyze dimensions, apparent crystallinity, crystal structure as well as the concentration of sulfate groups in the surface of the obtained CNCs were previously reported,38 and main results are collected in ESI Table S2.†Synthesis of poly(mannitol sebacate) (PMS) pre-polymer
Sebacic acid (SA) and mannitol (MA) were reacted in a manner described previously.16 Two poly(mannitol sebacate) pre-polymers were first synthesized to obtain 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 mannitol
2 mannitol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sebacic acid ratios. Poly(mannitol sebacate) pre-polymer with a mannitol
sebacic acid ratios. Poly(mannitol sebacate) pre-polymer with a mannitol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sebacic acid 1
sebacic acid 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio was prepared following a previously described procedure.38 To obtain the pre-polymer with a mannitol
1 ratio was prepared following a previously described procedure.38 To obtain the pre-polymer with a mannitol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sebacic acid 1
sebacic acid 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio,43 appropriated molar amounts (0.034
2 ratio,43 appropriated molar amounts (0.034![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.069) of MA (6.21 g) and SA (13.79 g) were charged into a 250 mL three-necked round-bottom flask equipped with a stirrer and a condenser, which was placed in an oil heating bath and purged for 0.5 h with nitrogen. The temperature was slowly increased to 150 °C under continuous stirring and nitrogen flow to produce approximately 20 g of pre-polymer (Scheme 1). The reaction was stopped after 5 h (1 h before gelation occurs), and the pre-polymer was dissolved in DMF (150 mg mL−1), filtered and purified by dropwise precipitation into a four-fold excess of cold ultrapure water under continuous stirring. The precipitated pre-polymer was collected and dried under vacuum until no more solvent was detected in the infrared spectra. The yield of the reaction for 1
0.069) of MA (6.21 g) and SA (13.79 g) were charged into a 250 mL three-necked round-bottom flask equipped with a stirrer and a condenser, which was placed in an oil heating bath and purged for 0.5 h with nitrogen. The temperature was slowly increased to 150 °C under continuous stirring and nitrogen flow to produce approximately 20 g of pre-polymer (Scheme 1). The reaction was stopped after 5 h (1 h before gelation occurs), and the pre-polymer was dissolved in DMF (150 mg mL−1), filtered and purified by dropwise precipitation into a four-fold excess of cold ultrapure water under continuous stirring. The precipitated pre-polymer was collected and dried under vacuum until no more solvent was detected in the infrared spectra. The yield of the reaction for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio was ∼82%, while for 1
1 ratio was ∼82%, while for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio was ∼88%, as calculated from the weight of the monomers before reaction and the weight of each obtained pre-polymer after reaction.
2 ratio was ∼88%, as calculated from the weight of the monomers before reaction and the weight of each obtained pre-polymer after reaction.
Characterization of PMS pre-polymers
The 1H-NMR spectrum of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 MA
2 MA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SA ratio PMS pre-polymers were acquired in DMSO-d6 on a Varian Mercury VX-300 MHz NMR spectrometer (ESI Fig. S1†). The compositions were determined from the ratio of the integrals of the signals associated with the mannitol and sebacic acid peaks16 which reveal an incorporation of stoichiometric ratios mannitol
SA ratio PMS pre-polymers were acquired in DMSO-d6 on a Varian Mercury VX-300 MHz NMR spectrometer (ESI Fig. S1†). The compositions were determined from the ratio of the integrals of the signals associated with the mannitol and sebacic acid peaks16 which reveal an incorporation of stoichiometric ratios mannitol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sebacic acid approximately of 1
sebacic acid approximately of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (ESI Table S1†).
2 (ESI Table S1†).
Preparation of PMS/CNC nanocomposites
The pre-polymers (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 MA
2 MA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SA ratio) were first dissolved in DMF (100 mg mL−1) by stirring at room temperature for 4 h. Suspensions of CNCs at concentration of 10 mg mL−1 were prepared at appropriate ratio in DMF (designed to combine with 1.5 g of dry pre-polymers and form 1 wt%, 5 wt% and 10 wt% of PMS/CNC nanocomposites) by 40 min of ultrasonic treatment in a horn sonicator (Q500 sonicator, QSonica) with 1 s on/off pulse conditions at a 20% amplitude. CNCs suspensions were stirred with a PMS pre-polymer/DMF solution for 30 min and cast into aluminium Petri dishes (93 × 7 mm) and allowed to dry in an oven at 70 °C. Films consisting of the neat pre-polymers were prepared for reference purposes under the same conditions. The resulting pre-polymer/nanocomposites and the neat pre-polymer films were placed in a vacuum oven for further reaction. Two different curing profiles were applied to both pre-polymer/nanocomposites series (1
SA ratio) were first dissolved in DMF (100 mg mL−1) by stirring at room temperature for 4 h. Suspensions of CNCs at concentration of 10 mg mL−1 were prepared at appropriate ratio in DMF (designed to combine with 1.5 g of dry pre-polymers and form 1 wt%, 5 wt% and 10 wt% of PMS/CNC nanocomposites) by 40 min of ultrasonic treatment in a horn sonicator (Q500 sonicator, QSonica) with 1 s on/off pulse conditions at a 20% amplitude. CNCs suspensions were stirred with a PMS pre-polymer/DMF solution for 30 min and cast into aluminium Petri dishes (93 × 7 mm) and allowed to dry in an oven at 70 °C. Films consisting of the neat pre-polymers were prepared for reference purposes under the same conditions. The resulting pre-polymer/nanocomposites and the neat pre-polymer films were placed in a vacuum oven for further reaction. Two different curing profiles were applied to both pre-polymer/nanocomposites series (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 MA
2 MA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SA reaction ratios), in which temperature and duration of the thermal treatment were varied to obtain samples with low and high degree of crosslinking and reaction ratios 1
SA reaction ratios), in which temperature and duration of the thermal treatment were varied to obtain samples with low and high degree of crosslinking and reaction ratios 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. Samples with low degree of crosslinking were maintained at 120 °C for 72 hours under vacuum (60 cm Hg). Samples with high degree of crosslinking were obtained by using the same protocol and subsequently increasing the temperature to 170 °C while maintaining the vacuum (60 cm Hg) for a further 24 hours. Both procedures afforded films of ∼150–200 μm of thickness.
2. Samples with low degree of crosslinking were maintained at 120 °C for 72 hours under vacuum (60 cm Hg). Samples with high degree of crosslinking were obtained by using the same protocol and subsequently increasing the temperature to 170 °C while maintaining the vacuum (60 cm Hg) for a further 24 hours. Both procedures afforded films of ∼150–200 μm of thickness.
Transmission electron microscopy (TEM)
CNCs TEM micrographs were recorded in a Phillips CM10 microscope with an accelerating voltage of 80 kV. Samples were prepared by drying a drop of dilute CNCs suspension in H2O (0.1 mg mL−1) onto a carbon-coated copper grid (Electron Microscopy Sciences) and subsequently dried under a heat lamp for 1 h.Differential scanning calorimetry (DSC)
Thermal behaviour of neat 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 PMS and their CNC nanocomposites was studied in a Mettler-Toledo DSC 800. Samples were heated from −60 to 180 °C, cooled down to −60 °C and heated again to 180 °C at a heating/cooling rate of 10 °C min−1 under an N2 atmosphere. The glass transition temperatures (Tg) were calculated as the midpoint of the transition in the second heating run for all samples.
2 PMS and their CNC nanocomposites was studied in a Mettler-Toledo DSC 800. Samples were heated from −60 to 180 °C, cooled down to −60 °C and heated again to 180 °C at a heating/cooling rate of 10 °C min−1 under an N2 atmosphere. The glass transition temperatures (Tg) were calculated as the midpoint of the transition in the second heating run for all samples.
Thermo gravimetric analysis (TGA)
Thermal stability of all obtained samples and CNCs was recorded in a Mettler-Toledo TGA/SDTA 851 modulus analyser. The samples (5–10 mg) were weighed in zirconia crucibles and were heated in air at a rate of 10 °C min−1 from ambient temperature to 700 °C. Peak degradation temperatures were determined using the first derivative curve to determine the onset of thermal degradation.Swelling and degradation studies in SBF
The swelling degree and degradation studies of all obtained samples (n = 4, 5 × 5 mm, 150–200 μm thickness) were studied under physiological conditions, in phosphate buffered saline solution (SBF) of 7.4 ± 0.5 at 37 °C, prepared following a previously reported protocol.46 For swelling studies, samples were collected after a 24 hour hydration period in SBF, blotted with a filter paper to remove excess surface water, and weighed (swelled weight; Ws). The swelling percentage of the networks after 24 h was determined comparing the swelled weight (Ws) with the initial weight (Wo) using eqn (1).
 | (1) |
The degradation behaviour was determined by monitoring the weight loss at different times. Samples were weighed, immersed in 15 mL of SBF and incubated at 37 °C in an oven for various periods of time. Polymer samples were removed from SBF at different time intervals (14 days, 28 days, 42 days, 56 days and 150 days), thoroughly washed with distilled H2O and dried under vacuum at 37 °C until constant weight was reached. The degradation degree of the polymers in SBF was calculated averaging the values of three samples by comparing the mass at each time interval (Mt) with the initial mass (Mo) using eqn (2),
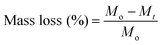 | (2) |
Fourier transform infrared spectroscopy (FTIR)
FTIR transmission spectra were recorded for all neat 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 PMS and their CNC nanocomposites, using a Thermo Nicolet 5700 spectrometer, between 500–4500 cm−1 with a 4 cm−1 resolution and an Attenuated Total Reflectance (ATR) cell. Backgrounds were acquired before every 3rd sample. All samples were vacuum-dried before measurement. FTIR spectra of neat 1
2 PMS and their CNC nanocomposites, using a Thermo Nicolet 5700 spectrometer, between 500–4500 cm−1 with a 4 cm−1 resolution and an Attenuated Total Reflectance (ATR) cell. Backgrounds were acquired before every 3rd sample. All samples were vacuum-dried before measurement. FTIR spectra of neat 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 PMS with low and high crosslinking degree samples after 150 days of immersion in SBF were also recorded.
2 PMS with low and high crosslinking degree samples after 150 days of immersion in SBF were also recorded.
Mechanical micro-testing
Tensile tests were carried out on a DEBEN microtester equipped with a 150 N load cell and operated at a crosshead speed of 0.4 mm min−1 at room temperature. Specimen dimensions were typically 15 × 4 mm × 150–200 μm. The elongation to break, Young's modulus and ultimate tensile strength were analysed for samples at initial stage (day 0 of degradation). Young's modulus of degraded samples was analysed at different degradation stages (immersion in SBF at 37 °C for 1, 28 and 150 days). Degraded samples were taken out from SBF and immediately tested. The Young's modulus (E) was determined from the initial slope of the stress–strain curves in the strain range of 0–10% for samples with low degree of crosslinking and 0–5% for samples with high degree of crosslinking. For each sample, a minimum of 5 rectangular samples were tested and the mechanical data were averaged.Results and discussion
Characterization of initial samples
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 MA
2 MA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SA reaction ratios) were achieved in a two-step process. Soluble poly(mannitol sebacate) pre-polymers with stoichiometric ratios of mannitol
SA reaction ratios) were achieved in a two-step process. Soluble poly(mannitol sebacate) pre-polymers with stoichiometric ratios of mannitol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sebacic acid 1
sebacic acid 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 were formed via the polycondensation reaction between the appropriated molar amounts of sebacic acid and D-mannitol (Scheme 1). In this first stage, the esterification is preferentially dominated by the reaction of the primary hydroxyl groups from D-mannitol (situated at both ends) with the carboxylic acid groups from sebacic acid.47,48 The reactions were stopped before gelation occurred, thus affording two polyesters with approximately the initial molar amounts incorporated during synthesis as was revealed by 1H NMR spectra (ESI Fig. S1†). Nanocomposites with 1 wt%, 5 wt%, and 10 wt% CNC were subsequently prepared by solution-casting mixtures of the PMS1
2 were formed via the polycondensation reaction between the appropriated molar amounts of sebacic acid and D-mannitol (Scheme 1). In this first stage, the esterification is preferentially dominated by the reaction of the primary hydroxyl groups from D-mannitol (situated at both ends) with the carboxylic acid groups from sebacic acid.47,48 The reactions were stopped before gelation occurred, thus affording two polyesters with approximately the initial molar amounts incorporated during synthesis as was revealed by 1H NMR spectra (ESI Fig. S1†). Nanocomposites with 1 wt%, 5 wt%, and 10 wt% CNC were subsequently prepared by solution-casting mixtures of the PMS1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and PMS1
1 and PMS1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 pre-polymers and the CNCs were cured in a second step under vacuum using two different curing profiles designed to prepare materials with low and high degree of crosslinking of each stoichiometric ratio, referred to in this paper as L and H, respectively. Fig. 1 and ESI Table S2† show TEM micrographs and the key properties of isolated CNCs used to develop the nanocomposites.
2 pre-polymers and the CNCs were cured in a second step under vacuum using two different curing profiles designed to prepare materials with low and high degree of crosslinking of each stoichiometric ratio, referred to in this paper as L and H, respectively. Fig. 1 and ESI Table S2† show TEM micrographs and the key properties of isolated CNCs used to develop the nanocomposites.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometric ratios prepared under a low and high degree of crosslinking conditions were recorded. Fig. 2 shows the most relevance bands for the neat PMS and PMS/CNC nanocomposite stoichiometric ratio 1
2 stoichiometric ratios prepared under a low and high degree of crosslinking conditions were recorded. Fig. 2 shows the most relevance bands for the neat PMS and PMS/CNC nanocomposite stoichiometric ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, respectively. The neat PMS samples show the characteristic absorption bands for hydrogen-bonded hydroxyl groups (3500–3200 cm−1) and for carbonyl-stretching vibrations of the ester groups (1800–1600 cm−1) in the polymer backbone, thus confirming the formation of polyesters. The bands around 2924 cm−1 and 2852 cm−1 are assigned to methylene (–CH2–) groups for the diacid residue and are observed in all spectra. A peak close to 1150 cm−1 is assigned to the –CO stretch associated with the ester groups (ESI Fig. S2†).49 Samples prepared under a low degree of crosslinking conditions (L-crosslinked) for both stoichiometric ratios showed a broad and intense –OH stretch peak at 3350 cm−1, an acid peak at 1705 cm−1, and an ester peak at 1740 cm−1, thus revealing a substantial fraction of unreacted hydroxyl and acid groups. The –OH band intensity was lower for the 1
2, respectively. The neat PMS samples show the characteristic absorption bands for hydrogen-bonded hydroxyl groups (3500–3200 cm−1) and for carbonyl-stretching vibrations of the ester groups (1800–1600 cm−1) in the polymer backbone, thus confirming the formation of polyesters. The bands around 2924 cm−1 and 2852 cm−1 are assigned to methylene (–CH2–) groups for the diacid residue and are observed in all spectra. A peak close to 1150 cm−1 is assigned to the –CO stretch associated with the ester groups (ESI Fig. S2†).49 Samples prepared under a low degree of crosslinking conditions (L-crosslinked) for both stoichiometric ratios showed a broad and intense –OH stretch peak at 3350 cm−1, an acid peak at 1705 cm−1, and an ester peak at 1740 cm−1, thus revealing a substantial fraction of unreacted hydroxyl and acid groups. The –OH band intensity was lower for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometric ratio while the acid peak (1705 cm−1) was more evident in accordance with the higher sebacic acid content and lower mannitol presence compared with the 1
2 stoichiometric ratio while the acid peak (1705 cm−1) was more evident in accordance with the higher sebacic acid content and lower mannitol presence compared with the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. All samples prepared under a high degree of crosslinking conditions (H-crosslinked) showed a reduction absorption spectra related to the hydroxyls groups at 3350 cm−1 a more distinct 1
1 ratio. All samples prepared under a high degree of crosslinking conditions (H-crosslinked) showed a reduction absorption spectra related to the hydroxyls groups at 3350 cm−1 a more distinct 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio, and a more pronounced –CO stretch associated with the ester groups, thus indicating a higher degree of esterification between acid and hydroxyl groups. The FTIR spectra of the nanocomposites revealed a similar pattern; it was able to discerned peaks associated with the CNC–OH close to 3338 cm−1 in the samples with 5 and 10 wt% CNCs.
2 ratio, and a more pronounced –CO stretch associated with the ester groups, thus indicating a higher degree of esterification between acid and hydroxyl groups. The FTIR spectra of the nanocomposites revealed a similar pattern; it was able to discerned peaks associated with the CNC–OH close to 3338 cm−1 in the samples with 5 and 10 wt% CNCs.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with a low degree of crosslinking had average UTS of 1.2 ± 0.7 MPa and a Young's modulus of 1.8 ± 0.3 MPa, while for the high crosslinking degree these values increase to 7.0 ± 0.6 MPa and 54.4 ± 3.3 MPa, respectively. While doubling the feed ratio of the sebacic acid monomer, the crosslink density increased30 and for low degree of crosslinking samples a four-fold enhancement in UTS (4.5 ± 0.7 MPa) and Young's modulus (7.2 ± 0.1 MPa) values were obtained compared with 1
1 with a low degree of crosslinking had average UTS of 1.2 ± 0.7 MPa and a Young's modulus of 1.8 ± 0.3 MPa, while for the high crosslinking degree these values increase to 7.0 ± 0.6 MPa and 54.4 ± 3.3 MPa, respectively. While doubling the feed ratio of the sebacic acid monomer, the crosslink density increased30 and for low degree of crosslinking samples a four-fold enhancement in UTS (4.5 ± 0.7 MPa) and Young's modulus (7.2 ± 0.1 MPa) values were obtained compared with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry. Elongation at break of these samples (low crosslinking degree) is also hardly affected by changing stoichiometry to 1
1 stoichiometry. Elongation at break of these samples (low crosslinking degree) is also hardly affected by changing stoichiometry to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio being reduced by half. In contrast, high degree of crosslinking 1
2 ratio being reduced by half. In contrast, high degree of crosslinking 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 PMS samples are the stiffer and stronger showing a higher Young's modulus for a comparable elongation to their 1
2 PMS samples are the stiffer and stronger showing a higher Young's modulus for a comparable elongation to their 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PMS counterpart, however, the enhancement in the mechanical properties comparing neat matrices with this crosslinking profile for both stoichiometric ratios, is not as marked as occurs for low degree of crosslinking samples. Thus altering monomer-feed ratio of sebacic acid (increasing it) in PMS elastomers resulted in a wide range of mechanical properties, whilst for high degree of crosslinking samples similar elongation at break is maintained with a slightly increase in the mechanical properties.
1 PMS counterpart, however, the enhancement in the mechanical properties comparing neat matrices with this crosslinking profile for both stoichiometric ratios, is not as marked as occurs for low degree of crosslinking samples. Thus altering monomer-feed ratio of sebacic acid (increasing it) in PMS elastomers resulted in a wide range of mechanical properties, whilst for high degree of crosslinking samples similar elongation at break is maintained with a slightly increase in the mechanical properties.
| Curing conditions, physical and mechanical properties of PMS and nanocomposites with low degree of crosslinking (L); 120 °C, 60 cm Hg, 3 days | ||||||||
|---|---|---|---|---|---|---|---|---|
| Sample | Mannitol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sebacic acid ratio sebacic acid ratio |
Young's modulusa (MPa) | Ultimate tensile strengtha (MPa) | Elongation at breaka (%) | Tg by DSCb (°C) | Degradation onset temperaturec (°C) | Toughnessa (MJ m−3) | Hydration by mass (%) |
| a Young's modulus was calculated from the initial slope between 0–10% of strain for low crosslinked samples and between 0–5% strain for high crosslinked samples. All the values are an average of 2–5 specimens and are determined from stress strain curves.b DSC glass transition temperatures were calculated as the midpoint of the transition in the 2nd heating run from differential scanning calorimetry traces (see ESI, Fig. S3).c Determined from thermogravimetric analysis derivative peak.d These samples break in the clamp due to the high stiffness. | ||||||||
| Neat PMS | Ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.8 ± 0.3 | 1.2 ± 0.7 | 80.0 ± 29.0 | 18 | 227 | 63.6 ± 50.0 | 19.6 ± 1.5 |
| PMS/1% CNC | 2.1 ± 0.5 | 2.3 ± 0.02 | 147.0 ± 31.1 | 18 | 237 | 187.4 ± 46.3 | 20.2 ± 0.2 | |
| PMS/5% CNC | 3.0 ± 0.3 | 4.6 ± 0.6 | 166.0 ± 20.5 | 18 | 230 | 393.5 ± 5.2 | 19.3 ± 1.1 | |
| PMS/10% CNC | 6.0 ± 0.7 | 5.6 ± 0.4 | 119.5 ± 18.3 | 17 | 217 | 389.0 ± 86.4 | 21.5 ± 0.3 | |
| Neat PMS | Ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
7.2 ± 0.1 | 4.5 ± 0.7 | 35.8 ± 3.5 | 16 | 238 | 81.8 ± 5.4 | 14.4 ± 1.0 |
| PMS/1% CNC | 11.1 ± 0.6 | 4.6 ± 1.2 | 25.6 ± 2.5 | 16 | 239 | 72.5 ± 22.6 | 12.0 ± 0.3 | |
| PMS/5% CNC | 14.8 ± 1.2 | 6.2 ± 1.3 | 25.4 ± 2.4 | 10 | 234 | 94.6 ± 23.5 | 11.4 ± 0.3 | |
| PMS/10% CNC | 32.3 ± 2.6 | 8.5 ± 2.0 | 23.4 ± 0.04 | 8 | 233 | 89.3 ± 39.1 | 10.4 ± 0.8 | |
| Curing conditions, physical and mechanical properties of PMS and nanocomposites with high degree of crosslinking (H); L + 170 °C, 60 cm Hg, 1 day | ||||||||
|---|---|---|---|---|---|---|---|---|
| Sample | Mannitol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sebacic acid ratio sebacic acid ratio |
Young's modulusa (MPa) | Ultimate tensile strengtha (MPa) | Elongation at breaka (%) | Tg by DSCb (°C) | Degradation onset temperaturec (°C) | Toughnessa (MJ m−3) | Hydration by mass (%) |
| Neat PMS | Ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
54.4 ± 3.3 | 7.0 ± 0.6 | 40.5 ± 7.0 | 26 | 263 | 204.4 ± 6.3 | 9.0 ± 0.2 |
| PMS/1% CNC | 54.5 ± 1.6 | 13.2 ± 2.2 | 94.1 ± 14.0 | 24 | 280 | 757.4 ± 238.0 | 8.7 ± 0.1 | |
| PMS/5% CNC | 132.5 ± 20.6 | 20.1 ± 3.4 | 77.0 ± 12.6 | 21 | 274 | 1047.0 ± 310.0 | 8.0 ± 0.3 | |
| PMS/10% CNC | 103.0 ± 12.2 | 19.4 ± 2.4 | 37.4 ± 6.0 | 20 | 268 | 470.0 ± 102.2 | 11.2 ± 0.5 | |
| Neat PMS | Ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
69.8 ± 0.6 | 13.3 ± 1.6 | 55.1 ± 4.9 | 32 | 271 | 482.0 ± 13.3 | 5.1 ± 0.8 |
| PMS/1% CNC | 146.4 ± 5.5 | 14.8 ± 3.3 | 35.4 ± 9.4 | 30 | 272 | 325.3 ± 9.9 | 4.7 ± 0.4 | |
| dPMS/5% CNC | 166.5 ± 4.0 | — | — | 23 | 277 | — | 2.2 ± 1.0 | |
| dPMS/10% CNC | 167.5 ± 5.0 | — | — | 21 | 278 | — | 2.1 ± 0.5 | |
Regarding nanocomposites, the improvement of the Young's modulus and tensile strength by increasing the CNCs content (Table 1) was appreciated for both stoichiometric ratios and crosslinking profiles. As was happened in previously reported 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PMS nanocomposites system, by applying the high crosslinking degree profile to 1
1 PMS nanocomposites system, by applying the high crosslinking degree profile to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 PMS (Fig. 3) nanocomposites results in much stiffer and stronger samples than their low crosslinking degree counterparts, reaching the highest Young's modulus close to 170 MPa for 5 and 10 wt% of CNC loads. Same CNCs content, produce in all 1
2 PMS (Fig. 3) nanocomposites results in much stiffer and stronger samples than their low crosslinking degree counterparts, reaching the highest Young's modulus close to 170 MPa for 5 and 10 wt% of CNC loads. Same CNCs content, produce in all 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 PMS nanocomposites a five-fold and nearly two-fold increase in Young's modulus, for low and high degree of crosslinking nanocomposites respectively, compared with the analogous samples of 1
2 PMS nanocomposites a five-fold and nearly two-fold increase in Young's modulus, for low and high degree of crosslinking nanocomposites respectively, compared with the analogous samples of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PMS nanocomposites. Thus, at equal CNCs load, the PMS 1
1 PMS nanocomposites. Thus, at equal CNCs load, the PMS 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 nanocomposites showed higher enhancement in mechanical properties than the PMS 1
2 nanocomposites showed higher enhancement in mechanical properties than the PMS 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 nanocomposites due to a higher crosslink density from the matrix. Surprisingly, for this stoichiometric ratio (1
1 nanocomposites due to a higher crosslink density from the matrix. Surprisingly, for this stoichiometric ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), the addition of a small amount of CNCs (only 1 wt%) results in a noticeable increase in the Young's modulus of 54% and 109% for low and high crosslinking degree samples, respectively, over the neat matrix. However, the addition of 1 wt% of CNCs in the 1
2), the addition of a small amount of CNCs (only 1 wt%) results in a noticeable increase in the Young's modulus of 54% and 109% for low and high crosslinking degree samples, respectively, over the neat matrix. However, the addition of 1 wt% of CNCs in the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio does not produce as high reinforcement as in the 1
1 ratio does not produce as high reinforcement as in the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 PMS matrix, thus having similar Young's modulus to the neat polymer.
2 PMS matrix, thus having similar Young's modulus to the neat polymer.
Another remarkable effect reported in the previous work,38 and in what differs the behaviour of nanocomposites with different stoichiometry, was that increasing the CNCs content in the PMS 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 samples provided higher stiffness without significantly compromising the elongation at break of the composites for both crosslinking profiles. This was related to a molecular level reinforcement induced by reactions between the nanoparticles with high hydroxyl functionality and the matrix during the second polycondensation step, which also occur for previously reported clays and CNC-reinforced polyurethane nanocomposites.50–52 In contrast increasing the CNCs content in PMS 1
1 samples provided higher stiffness without significantly compromising the elongation at break of the composites for both crosslinking profiles. This was related to a molecular level reinforcement induced by reactions between the nanoparticles with high hydroxyl functionality and the matrix during the second polycondensation step, which also occur for previously reported clays and CNC-reinforced polyurethane nanocomposites.50–52 In contrast increasing the CNCs content in PMS 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 matrix for both crosslinking profiles, results in higher stiffness whereas the elongation at break decreases, but not significantly. Therefore, the increase in mechanical properties without compromising the elongation at break in the 1
2 matrix for both crosslinking profiles, results in higher stiffness whereas the elongation at break decreases, but not significantly. Therefore, the increase in mechanical properties without compromising the elongation at break in the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 system are due to the presence of CNC nanoparticles in the matrix and from polymer molecules interacting physically and chemically with CNC surfaces; however, for the 1
1 system are due to the presence of CNC nanoparticles in the matrix and from polymer molecules interacting physically and chemically with CNC surfaces; however, for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 system, the higher improvement in the mechanical properties, even for lower CNC contents, and the slight decrease in elongation at break, seem to be influenced by the higher crosslink density, together with the presence of well-dispersed stiff nanoparticles and CNC/CNC interactions, more than from polymer/CNC interactions.
2 system, the higher improvement in the mechanical properties, even for lower CNC contents, and the slight decrease in elongation at break, seem to be influenced by the higher crosslink density, together with the presence of well-dispersed stiff nanoparticles and CNC/CNC interactions, more than from polymer/CNC interactions.
The different behaviors observed in terms of stiffness, strength, and elongation at break between the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 neat matrices with the CNCs presence can be associated to the differences in the OH
1 neat matrices with the CNCs presence can be associated to the differences in the OH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) COOH ratio. One must consider that CNC is highly –OH functional and capable to interact with the residual hydroxyl or acid groups from PMS. As was already reported, as the value of average functionality (fav) increases, the degree of polymerization also increases.47,53 For the 1
COOH ratio. One must consider that CNC is highly –OH functional and capable to interact with the residual hydroxyl or acid groups from PMS. As was already reported, as the value of average functionality (fav) increases, the degree of polymerization also increases.47,53 For the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PMS, and 1
1 PMS, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 PMS, the fav calculated using Pinner's equation53,54 was 1.3 and 2.6, respectively. This implies that for the 1
2 PMS, the fav calculated using Pinner's equation53,54 was 1.3 and 2.6, respectively. This implies that for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 pre-polymer, the free reactive sites available after the first polycondensation step are probably lower than in the 1
2 pre-polymer, the free reactive sites available after the first polycondensation step are probably lower than in the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 pre-polymer due to the higher density of crosslinking that results in fewer possibilities to form PMS/CNC interactions during the extent of the second polycondensation step. In the same way, the high offset in stoichiometry between available carboxylic acid and hydroxyl groups, is also responsible of losing the positive effect in the mechanical properties and elongation at break (tends to decrease) for the PMS 1
1 pre-polymer due to the higher density of crosslinking that results in fewer possibilities to form PMS/CNC interactions during the extent of the second polycondensation step. In the same way, the high offset in stoichiometry between available carboxylic acid and hydroxyl groups, is also responsible of losing the positive effect in the mechanical properties and elongation at break (tends to decrease) for the PMS 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 10 wt% of CNC nanocomposite with a high degree of crosslinking, as produce a loss of the polymer–filler synergistic effect.38,55
1 and 10 wt% of CNC nanocomposite with a high degree of crosslinking, as produce a loss of the polymer–filler synergistic effect.38,55
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 reaction ratios. Samples with a higher sebacic acid ratio have a lower swelling degree that reveals an increase of the crosslink density of the networks for the same curing conditions in agreement with the mechanical property results.30 For example, the neat PMS 1
2 reaction ratios. Samples with a higher sebacic acid ratio have a lower swelling degree that reveals an increase of the crosslink density of the networks for the same curing conditions in agreement with the mechanical property results.30 For example, the neat PMS 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 obtained with low crosslinking curing conditions, swells to 17.7 ± 1.0% and to 8.7 ± 0.5% cured with high crosslinking conditions, whereas changing the reaction ratio to 1
1 obtained with low crosslinking curing conditions, swells to 17.7 ± 1.0% and to 8.7 ± 0.5% cured with high crosslinking conditions, whereas changing the reaction ratio to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 reduces the swelling degree to 14.4 ± 1.0% and to 5.1 ± 0.8 for the low and high crosslinking degree of the neat PMS, respectively. Moreover, it seems that there are two clearly differentiated effects with the addition of CNCs to the 1
2 reduces the swelling degree to 14.4 ± 1.0% and to 5.1 ± 0.8 for the low and high crosslinking degree of the neat PMS, respectively. Moreover, it seems that there are two clearly differentiated effects with the addition of CNCs to the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 neat PMS networks. In the case of the 1
2 neat PMS networks. In the case of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, the addition of CNCs does not seem to significantly modify the swelling behavior of the neat matrix for low crosslinking degree nanocomposites, while it tends to slightly decrease for 1 and 5 wt% of CNC for high crosslinking degree samples. High crosslinking degree samples with 10 wt% of CNCs have the highest swelling degree; this fact is in concordance with the observed mechanical behavior and could be ascribed to the loss of polymer–filler interactions due to the high offset in the stoichiometry. For 1
1 ratio, the addition of CNCs does not seem to significantly modify the swelling behavior of the neat matrix for low crosslinking degree nanocomposites, while it tends to slightly decrease for 1 and 5 wt% of CNC for high crosslinking degree samples. High crosslinking degree samples with 10 wt% of CNCs have the highest swelling degree; this fact is in concordance with the observed mechanical behavior and could be ascribed to the loss of polymer–filler interactions due to the high offset in the stoichiometry. For 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometric, it is more evident that the addition of CNCs results in a lower swelling degree for both curing conditions. The addition of 10 wt% of CNCs reduces the swelling degree to 10.4 ± 0.8 and 2.1 ± 0.5 for low and high crosslinking degree samples respectively. These results indicate that for the 1
2 stoichiometric, it is more evident that the addition of CNCs results in a lower swelling degree for both curing conditions. The addition of 10 wt% of CNCs reduces the swelling degree to 10.4 ± 0.8 and 2.1 ± 0.5 for low and high crosslinking degree samples respectively. These results indicate that for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio samples, the crosslinking degree of 1 and 5 wt% CNC content nanocomposites, is mainly governed by the PMS matrix and CNC interactions, probably due to the excess of hydroxyl groups in the polymer backbone and the new introduction by the fillers, while higher CNC contents (10 wt%) offer an excess of hydroxyl groups that would favour the CNC/CNC interactions, thus reducing the polymer/polymer interactions and, hence, the crosslinking degree. In the case of samples with a higher sebacic acid feed ratio, the excess acid groups, instead of hydroxyl groups in the polymer backbone, are expected to provide additional esterification sites that, together with the hydroxyl groups available in the CNCs, seem to be the responsible for the reduction in the swelling degree with the increase of the CNCs content; that is, at least in a certain extent, CNC can act as a chemical crosslinker to enhance the crosslinking between CNC and PMS chains.56
1 ratio samples, the crosslinking degree of 1 and 5 wt% CNC content nanocomposites, is mainly governed by the PMS matrix and CNC interactions, probably due to the excess of hydroxyl groups in the polymer backbone and the new introduction by the fillers, while higher CNC contents (10 wt%) offer an excess of hydroxyl groups that would favour the CNC/CNC interactions, thus reducing the polymer/polymer interactions and, hence, the crosslinking degree. In the case of samples with a higher sebacic acid feed ratio, the excess acid groups, instead of hydroxyl groups in the polymer backbone, are expected to provide additional esterification sites that, together with the hydroxyl groups available in the CNCs, seem to be the responsible for the reduction in the swelling degree with the increase of the CNCs content; that is, at least in a certain extent, CNC can act as a chemical crosslinker to enhance the crosslinking between CNC and PMS chains.56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 PMS and PMS/CNC samples. In the case of thermal stability, the neat PMS and nanocomposites with a high degree of crosslinking revealed an increase of thermal stability for these curing conditions compared with their low crosslinking counterparts. Yet no marked differences in the thermal stability behavior were evidenced by comparing both reaction ratios (1
2 PMS and PMS/CNC samples. In the case of thermal stability, the neat PMS and nanocomposites with a high degree of crosslinking revealed an increase of thermal stability for these curing conditions compared with their low crosslinking counterparts. Yet no marked differences in the thermal stability behavior were evidenced by comparing both reaction ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). Some of the nanocomposites containing 5–10 wt% of CNCs showed a slightly lower degradation onset temperature than the neat PMS or 1 wt% CNC samples, most likely due to the lower onset degradation temperature associated with the CNC glycosyl units degradation starting at 220 °C.57 Regarding the DSC results, there are two factors in the nanocomposites that could influence Tg in opposite directions. First, for the same curing profile, all the high crosslinking degree samples had a Tg higher than the low crosslinking degree samples; that is, longer curing times and higher temperature result in a higher degree of crosslinking, probably due to that hydrogen bonding and/or chemical crosslink was gradually intensified during thermal curing, which hinders the mobility of polymeric chains and increases the Tg. Moreover, Tg tends to decrease by slightly increasing the CNCs content for the 1
2). Some of the nanocomposites containing 5–10 wt% of CNCs showed a slightly lower degradation onset temperature than the neat PMS or 1 wt% CNC samples, most likely due to the lower onset degradation temperature associated with the CNC glycosyl units degradation starting at 220 °C.57 Regarding the DSC results, there are two factors in the nanocomposites that could influence Tg in opposite directions. First, for the same curing profile, all the high crosslinking degree samples had a Tg higher than the low crosslinking degree samples; that is, longer curing times and higher temperature result in a higher degree of crosslinking, probably due to that hydrogen bonding and/or chemical crosslink was gradually intensified during thermal curing, which hinders the mobility of polymeric chains and increases the Tg. Moreover, Tg tends to decrease by slightly increasing the CNCs content for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 low crosslinking degree samples, thus becoming more prominent for high crosslinking and 1
1 low crosslinking degree samples, thus becoming more prominent for high crosslinking and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio samples due to the disruption of polymeric network.49,58 This second phenomenon could be explained as follows: in the first step of the condensation there occurs an esterification dominated by the reaction of the primary hydroxyl groups founded at both ends of mannitol with carboxylic acid groups from sebacic acid, thus leaving a large amount of secondary –OH groups in the pre-polymer backbone and also an excess of free carboxylic acid groups for 1
2 ratio samples due to the disruption of polymeric network.49,58 This second phenomenon could be explained as follows: in the first step of the condensation there occurs an esterification dominated by the reaction of the primary hydroxyl groups founded at both ends of mannitol with carboxylic acid groups from sebacic acid, thus leaving a large amount of secondary –OH groups in the pre-polymer backbone and also an excess of free carboxylic acid groups for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio. For both systems, CNCs were added into the PMS pre-polymers at this point and were subject to low and high crosslinking conditions, which considerably changed the OH/COOH ratio. These variations in the initial stoichiometry reaction of the 1
2 ratio. For both systems, CNCs were added into the PMS pre-polymers at this point and were subject to low and high crosslinking conditions, which considerably changed the OH/COOH ratio. These variations in the initial stoichiometry reaction of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratios not only seemed to be responsible for CNCs not increasing the Tg as was expected, but also shifting it to lower temperatures for nanocomposites.57 Similar behavior was observed for polyurethanes reinforced with CNCs and poly(glycerol sebacate) bioglass nanocomposites due to strong associations between the filler and matrix.50,58 The new interactions between polymer chains and CNCs which seem to be favoured by higher sebacic acid feed ratios, higher crosslinking conditions, and raising the filler contents, all of which could reduce the polymer/polymer interactions and, therefore, be responsible for lowering the Tg.
2 ratios not only seemed to be responsible for CNCs not increasing the Tg as was expected, but also shifting it to lower temperatures for nanocomposites.57 Similar behavior was observed for polyurethanes reinforced with CNCs and poly(glycerol sebacate) bioglass nanocomposites due to strong associations between the filler and matrix.50,58 The new interactions between polymer chains and CNCs which seem to be favoured by higher sebacic acid feed ratios, higher crosslinking conditions, and raising the filler contents, all of which could reduce the polymer/polymer interactions and, therefore, be responsible for lowering the Tg.Characterization of degraded samples
Fig. 4 shows the obtained degradation rates that seem to be loosely correlated to the swelling and mechanical properties. Taking these results into account, roughly, the highest degradation rates correspond to samples with the lowest mechanical properties and highest swelling degrees; thus, to the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 low crosslinking degree samples and nanocomposites. Continuing with the observed trend, the 1
1 low crosslinking degree samples and nanocomposites. Continuing with the observed trend, the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 reaction ratio samples with a high crosslinking degree have slightly slower degradation behavior than the 1
1 reaction ratio samples with a high crosslinking degree have slightly slower degradation behavior than the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 low crosslinking degree samples, while the slowest degradation rate was obtained for the high crosslinking degree 1
2 low crosslinking degree samples, while the slowest degradation rate was obtained for the high crosslinking degree 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 samples. Comparing both reaction ratios, an increase in sebacic acid content increases the time required for degradation due to an extent in the crosslink density that can effectively reduce the degradation rate for the same curing profile. Interestingly, the presence of CNCs does not seem to have a great influence on the degradation rate of the neat polymer matrices. As was observed in previously reported systems,60 the differences in mass loss between the two studied systems (1
2 samples. Comparing both reaction ratios, an increase in sebacic acid content increases the time required for degradation due to an extent in the crosslink density that can effectively reduce the degradation rate for the same curing profile. Interestingly, the presence of CNCs does not seem to have a great influence on the degradation rate of the neat polymer matrices. As was observed in previously reported systems,60 the differences in mass loss between the two studied systems (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) could also be related to the higher presence of mannitol, which is more noticeable in the 1
2) could also be related to the higher presence of mannitol, which is more noticeable in the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 low crosslinking degree samples, that increases the hydrolytic degradation rate and enhances water penetration into the polymer matrix. An increase in the crosslink density achieved by changing the mannitol
1 low crosslinking degree samples, that increases the hydrolytic degradation rate and enhances water penetration into the polymer matrix. An increase in the crosslink density achieved by changing the mannitol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sebacic acid ratio from 1
sebacic acid ratio from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (corresponding to more equal numbers of alcohol and carboxylic groups) was also reported to be an important factor to significantly decrease the mass loss.61 The results indicate considerable impact of the network structure on the degradation rates, which can be tuned or slowed by changing the stoichiometric ratio or crosslinking conditions without being affected by CNCs presence. To confirm the degradation of the samples, Fig. 5 shows FTIR spectra's main bands of films immersed in SBF after 150 days. All the samples developed a new peak centred around 1560 cm−1, which is not present in the spectra of dry samples. This peak is characteristic of the stretch of carboxylate groups, indicating that some salts of carboxylic acid have been formed due to the hydrolysed ester bonds.62,63
2 (corresponding to more equal numbers of alcohol and carboxylic groups) was also reported to be an important factor to significantly decrease the mass loss.61 The results indicate considerable impact of the network structure on the degradation rates, which can be tuned or slowed by changing the stoichiometric ratio or crosslinking conditions without being affected by CNCs presence. To confirm the degradation of the samples, Fig. 5 shows FTIR spectra's main bands of films immersed in SBF after 150 days. All the samples developed a new peak centred around 1560 cm−1, which is not present in the spectra of dry samples. This peak is characteristic of the stretch of carboxylate groups, indicating that some salts of carboxylic acid have been formed due to the hydrolysed ester bonds.62,63
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 low crosslinking degree samples still mostly retained their shape but had completely lost their mechanical compliance and their testing and manipulation became impractical. The mechanical behavior under hydrated conditions of biomaterials is an important factor in determining their possible biomedical and biological applications59 since a drastic reduction in the mechanical properties of conventional polyesters between dry and wet conditions, may induce an inflammatory response that enhances fibrous capsule formation.49 Regarding to the developed materials, it was observed that all the samples had a similar Young's modulus after one day hydration under physiological conditions that demonstrated low differences between dry and wet conditions. As expected, for the low and high crosslinking degree of neat PMS 1
1 low crosslinking degree samples still mostly retained their shape but had completely lost their mechanical compliance and their testing and manipulation became impractical. The mechanical behavior under hydrated conditions of biomaterials is an important factor in determining their possible biomedical and biological applications59 since a drastic reduction in the mechanical properties of conventional polyesters between dry and wet conditions, may induce an inflammatory response that enhances fibrous capsule formation.49 Regarding to the developed materials, it was observed that all the samples had a similar Young's modulus after one day hydration under physiological conditions that demonstrated low differences between dry and wet conditions. As expected, for the low and high crosslinking degree of neat PMS 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and its CNC nanocomposites, a prolonged incubation time of up to 30 days produced a significant drop in the Young's modulus compared with the 1
1 and its CNC nanocomposites, a prolonged incubation time of up to 30 days produced a significant drop in the Young's modulus compared with the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio samples due to the higher presence of the hydrophilic nature mannitol unit.59 In contrast, Young's modulus for the low and high crosslinking degree of 1
2 ratio samples due to the higher presence of the hydrophilic nature mannitol unit.59 In contrast, Young's modulus for the low and high crosslinking degree of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 PMS samples and CNC nanocomposites is less deteriorated over time in culture media. Similar behaviors have been reported for the poly(xylitol sebacate) 1
2 PMS samples and CNC nanocomposites is less deteriorated over time in culture media. Similar behaviors have been reported for the poly(xylitol sebacate) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratios elastomers.34 The dependence of Young's modulus on the immersion time in culture medium revealed marked differences between 1
2 ratios elastomers.34 The dependence of Young's modulus on the immersion time in culture medium revealed marked differences between 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratios without a clear dependence with CNC content, except for the high crosslinking degree of 1
2 ratios without a clear dependence with CNC content, except for the high crosslinking degree of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 PMS and nanocomposites. These samples are able to retain the initial stiffness by increasing the CNC content over the degradation time, probably due to less deterioration of the network and motivated by a higher crosslink density.
2 PMS and nanocomposites. These samples are able to retain the initial stiffness by increasing the CNC content over the degradation time, probably due to less deterioration of the network and motivated by a higher crosslink density.
In general, mechanical properties are expected to decrease with time for these systems; however, after long periods of incubation (150 days), the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 neat PMS and nanocomposites with a high degree of crosslinking had an increased Young's modulus, yet the other samples tended to decrease or remain unchanged after 30 days of incubation. Similar phenomena was observed in poly(propylene fumarate) composites and were initially ascribed to the complexation of the carboxylic groups formed during degradation.64,65 In our case, it could probably be related to the generation of new crosslinking/interaction points in the network due to the scission of ester linkages that increase free carboxylic acid salts (Fig. 5). The fact that it is only occurring for these samples poses the question of whether these new crosslinking/interaction points can be favoured for the higher network density, and within that, whether all the new available functional groups are closer and can easily interact.
2 neat PMS and nanocomposites with a high degree of crosslinking had an increased Young's modulus, yet the other samples tended to decrease or remain unchanged after 30 days of incubation. Similar phenomena was observed in poly(propylene fumarate) composites and were initially ascribed to the complexation of the carboxylic groups formed during degradation.64,65 In our case, it could probably be related to the generation of new crosslinking/interaction points in the network due to the scission of ester linkages that increase free carboxylic acid salts (Fig. 5). The fact that it is only occurring for these samples poses the question of whether these new crosslinking/interaction points can be favoured for the higher network density, and within that, whether all the new available functional groups are closer and can easily interact.
There are a couple of important features that can be concluded from this degradation study: on the one hand, the synthesized materials cover a wide range of mechanical properties and, hence, may be used for biomedical and tissue engineering. For example, tough living tissues such as tendon (E = 143–2310 MPa), human ligament (E = 65–541 MPa), or cancellous bone (E = 20–500 MPa)66 have a Young's modulus comparable to the high crosslinking degree samples. In applications where the involved tissue is softer and more elastic, the low crosslinking degree samples could be offered similar mechanical compliance for some human elastic soft tissues such as knee articular cartilage (2.1–11.8 MPa),67,68 cerebral vein (6.85 MPa) and artery (15.6 MPa), aortic valve leaflet (15 ± 6 MPa)59 or pericardium (20.4 ± 1.9 MPa).69 On the other hand, in the context of biomaterials applications, the design of mechanically stable materials over an extended period is of importance for tissue remodelling at the wound site.70,71 Regarding this highly desirable mechanical stability following implantation, the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 reaction ratio nanocomposites have the highest mechanical stability over a one month period, and could be good candidates for mechanical support to damaged tissues during the lag phase of the healing process. Finally, it should be pointed out that for long-term biomedical applications, one major requirement of any material is that must be biocompatible. Thus, it has to be able to stay in contact with living tissues without causing any cytotoxic or other derived side effects. Previous studies have investigated approaches to create PPS composites based on the introduction of inorganic components such as halloysite nanotubes (clays) or multi-walled carbon nanotubes (MWCNTs) with improved mechanical properties.70,72,73 However, the main drawback derived from the use of these inorganic nanoparticles in vivo is their potential toxicity as well as the uncertain (or non-existent) mechanism of removal from the body.74 By using organic based fillers these drawbacks could be lessen. In this regards, although CNC is known as non-biodegradable material for a long time, it has recently gained attention because exhibit low cytotoxicity with a range of animal and human cell types and no cytotoxicity at concentration ranges of ∼50 μg mL−1 of CNC.74–79 Mahmoud et al.80 reported that any noticeable cytotoxic effect on two different cell lines occurred during in vitro cellular studies of two differently charged CNCs (positively and negatively) suggesting possible applications in both drug delivery and bioimaging. Jia et al.81 reported potential application of microcrystal cellulose (MCC) and CNC as a filler of electrospun cellulose acetate vascular tissue scaffolds to improve biocompatibility. Thus, although the present work should be biocompatible, given that both components have been previously tested separately, future investigations are needed to characterize the toxicology of these cellulose nanocomposites materials thorough in vitro and in vivo testing, to confirm the potential biomedical application. The in vitro studies reported until now highlight that CNCs hold great promise in a wide variety of biotechnological and biomedical applications especially in tissue engineering.82
2 reaction ratio nanocomposites have the highest mechanical stability over a one month period, and could be good candidates for mechanical support to damaged tissues during the lag phase of the healing process. Finally, it should be pointed out that for long-term biomedical applications, one major requirement of any material is that must be biocompatible. Thus, it has to be able to stay in contact with living tissues without causing any cytotoxic or other derived side effects. Previous studies have investigated approaches to create PPS composites based on the introduction of inorganic components such as halloysite nanotubes (clays) or multi-walled carbon nanotubes (MWCNTs) with improved mechanical properties.70,72,73 However, the main drawback derived from the use of these inorganic nanoparticles in vivo is their potential toxicity as well as the uncertain (or non-existent) mechanism of removal from the body.74 By using organic based fillers these drawbacks could be lessen. In this regards, although CNC is known as non-biodegradable material for a long time, it has recently gained attention because exhibit low cytotoxicity with a range of animal and human cell types and no cytotoxicity at concentration ranges of ∼50 μg mL−1 of CNC.74–79 Mahmoud et al.80 reported that any noticeable cytotoxic effect on two different cell lines occurred during in vitro cellular studies of two differently charged CNCs (positively and negatively) suggesting possible applications in both drug delivery and bioimaging. Jia et al.81 reported potential application of microcrystal cellulose (MCC) and CNC as a filler of electrospun cellulose acetate vascular tissue scaffolds to improve biocompatibility. Thus, although the present work should be biocompatible, given that both components have been previously tested separately, future investigations are needed to characterize the toxicology of these cellulose nanocomposites materials thorough in vitro and in vivo testing, to confirm the potential biomedical application. The in vitro studies reported until now highlight that CNCs hold great promise in a wide variety of biotechnological and biomedical applications especially in tissue engineering.82
Conclusions
Several strategies have been employed to prepare biodegradable poly(mannitol sebacate) based materials having a broad range of mechanical properties and degradation characteristics. These strategies are based on varying feed reaction ratios, crosslinking conditions or the addition of CNCs as reinforcing fillers to alter the degradation rates as well as to tailor the mechanical stiffness of the poly(mannitol sebacate) from 1.8 ± 0.3 MPa to 167.5 ± 5.0 MPa. Compared to similar reported systems based in poly(glycerol sebacate), addition of high contents of nano-clays or Bioglass® (10–20 wt%) are necessary to achieve Young's modulus close to 2 MPa. In this regard, the addition of CNCs in poly(mannitol sebacate) matrices combined with different stoichiometry and crosslinking profiles, results in a more efficient and remarkable improvement in mechanical properties well above of the enhancement obtained for other fillers. Reported systems containing Bioglass® tends to experiment a sudden drop in mechanical properties after one day of immersion in SBF medium almost getting back to the strength level of neat materials. In comparison PMS/CNC nanocomposites, provides more reliable mechanical support materials without immediately loss of mechanical stability. The amorphous nature of materials prepared provides the retention of mechanical properties and shape stability during long periods of incubation which are beneficial as could allow the load to be transferred to the surrounding tissues considering the development of a possible biomedical device. Moreover, acceleration (or deceleration) of the mass loss in similar nanocomposites, have been typically achieved through varying the filler content, or crosslinking degree of the neat matrix, but to our knowledge, no studies about the affection of both variables together for the same nano-filler composition over the degradation behaviour have been reported. Mass lose rate was primary determined by the degree of the crosslink density of materials that demonstrate slower degradation with the increasing feed ratio of sebacic acid or thermal curing time and temperature. However, the presence of CNCs does not slow or accelerate the mass loss of the matrix, that makes easy to predict the behavior of the material over the degradation period. In summary, the large number of variables to be applied during the synthesis of these nanocomposites provides a wide range of possible mechanical properties, which could be broadly employed in many biomedical applications that approximate those of soft and tough tissues. Moreover, in vivo experiments will also provide the indications of biocompatibility, and finally determine the real utility of these materials.Acknowledgements
The authors gratefully acknowledge financial support received from Spanish Ministry of Economy and Competitiveness (Project MAT2010/21494-C03), as well as the support of FPU grant from MED (MED-FPU; AP2009-2482) and the Adolphe Merkle Foundation.References
- Z. Sun, C. Chen, M. Sun, C. Ai, X. Lu, Y. Zheng, B. Yang and D. Dong, Biomaterials, 2009, 30, 5209–5214 CrossRef CAS PubMed.
- C. Sundback, J. Shyu, Y. Wang, W. Faquin, R. Langer, J. Vacanti and T. Hadlock, Biomaterials, 2005, 26, 5454–5464 CrossRef CAS PubMed.
- D. Motlagh, J. Yang, K. Lui, A. Webb and G. Ameer, Biomaterials, 2006, 27, 4315–4324 CrossRef CAS PubMed.
- A. Mahdavi, L. Ferreira, C. Sundback, J. W. Nichol, E. P. Chan, D. J. D. Carter, C. J. Bettinger, S. Patanavanich, L. Chignozha, E. Ben-Joseph, A. Galakatos, H. Pryor, I. Pomerantseva, P. T. Masiakos, W. Faquin, A. Zumbuehl, S. Hong, J. Borenstein, J. Vacanti, R. Langer and J. M. Karp, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 2307–2312 CrossRef CAS PubMed.
- I. Vroman and L. Tighzert, Materials, 2009, 2, 307–344 CrossRef CAS PubMed.
- B. D. Ulery, L. S. Nair and C. T. Laurencin, J. Polym. Sci., Part B: Polym. Phys., 2011, 49, 832–864 CrossRef CAS PubMed.
- L. Xue and H. P. Greisler, J. Vasc. Surg., 2003, 37, 472–480 CrossRef PubMed.
- L. S. Nair and C. T. Laurencin, Prog. Polym. Sci., 2007, 32, 762–798 CrossRef CAS PubMed.
- P. A. Gunatillake and R. Adhikari, Eur. Cells Mater., 2003, 5, 1–16 CAS.
- J. C. Middleton and A. J. Tipton, Biomaterials, 2000, 21, 2335–2346 CrossRef CAS.
- K. Athanasiou, C. Agrawal, F. Barber and S. Burkhart, Arthroscopy, 1998, 14, 726–737 CrossRef CAS.
- I. Engelberg and J. Kohn, Biomaterials, 1991, 12, 292–304 CrossRef CAS.
- Q. Chen, A. Bismarck, U. Hansen, S. Junaid, M. Q. Tran, S. E. Harding, N. N. Ali and A. R. Boccaccini, Biomaterials, 2008, 29, 47–57 CrossRef CAS PubMed.
- S. Liang, W. D. Cook, G. A. Thouas and Q. Chen, Biomaterials, 2010, 31, 8516–8529 CrossRef CAS PubMed.
- M. A. Meyers, P. Chen, A. Y. Lin and Y. Seki, Prog. Mater. Sci., 2008, 53, 1–206 CrossRef CAS PubMed.
- J. P. Bruggeman, B. de Bruin, C. J. Bettinger and R. Langer, Biomaterials, 2008, 29, 4726–4735 CrossRef CAS PubMed.
- Y. Li, G. A. Thouas and Q. Chen, RSC Adv., 2012, 22, 8229–8242 RSC.
- J. Yang, A. Webb, S. Pickerill, G. Hageman and G. Ameer, Biomaterials, 2006, 27, 1889–1898 CrossRef CAS PubMed.
- J. Yang, A. Webb and G. Ameer, Adv. Mater., 2004, 16, 511–516 CrossRef CAS PubMed.
- H. Park, J. Seo, H. Lee, H. Kim, I. B. Wall, M. Gong and J. C. Knowles, Acta Biomater., 2012, 8, 2911–2918 CrossRef CAS PubMed.
- Z. Sun, L. Wu, X. Lu, Z. Meng, Y. Zheng and D. Dong, Appl. Surf. Sci., 2008, 255, 350–352 CrossRef CAS PubMed.
- Q. Liu, T. Tan, J. Weng and L. Zhang, Biomed. Mater., 2009, 4, 025015 CrossRef PubMed.
- M. Y. Krasko, A. Shikanov, A. Ezra and A. J. Domb, J. Polym. Sci., Part A: Polym. Chem., 2003, 41, 1059–1069 CrossRef CAS PubMed.
- P. S. Sathiskumar and G. Madras, Polym. Degrad. Stab., 2011, 96, 1695–1704 CrossRef CAS PubMed.
- I. Djordjevic, N. R. Choudhury, N. K. Dutta and S. Kumar, Polymer, 2009, 50, 1682–1691 CrossRef CAS PubMed.
- Y. Wang, G. Ameer, B. Sheppard and R. Langer, Nat. Biotechnol., 2002, 20, 602–606 CrossRef CAS PubMed.
- D. G. Barrett and M. N. Yousaf, Molecules, 2009, 14, 4022–4050 CrossRef CAS PubMed.
- K. Ellwood, Am. J. Clin. Nutr., 1995, 62, 1169–1174 Search PubMed.
- S. Natah, K. Hussien, J. Tuominen and V. Koivisto, Am. J. Clin. Nutr., 1997, 65, 947–950 CAS.
- J. P. Bruggeman, C. J. Bettinger, C. L. E. Nijst, D. S. Kohane and R. Langer, Adv. Mater., 2008, 20, 1922–1927 CrossRef CAS PubMed.
- S. Liang, W. D. Cook, G. A. Thouas and Q. Chen, Biomaterials, 2010, 31, 8516–8529 CrossRef CAS PubMed.
- C. Bettinger, E. Weinberg, K. Kulig, J. Vacanti, Y. Wang, J. Borenstein and R. Langer, Adv. Mater., 2006, 18, 165–169 CrossRef CAS PubMed.
- Y. Wang, Y. Kim and R. Langer, J. Biomed. Mater. Res., Part A, 2003, 66, 192–197 CrossRef PubMed.
- J. P. Bruggeman, C. J. Bettinger and R. Langer, J. Biomed. Mater. Res., Part A, 2010, 95, 92–104 CrossRef PubMed.
- K. Firoozbakhsh, I. Yi, M. Moneim and Y. Umada, Clin. Orthop., 2002, 240–247 CrossRef PubMed.
- S. K. Ha, Med. Eng. Phys., 2006, 28, 534–541 CrossRef PubMed.
- V. R. Sastri, Plastics in Medical Devices, William Andrew Publishing, Boston, 2nd edn., 2010, pp. 217–262 Search PubMed.
- A. Sonseca, S. Camarero-Espinosa, L. Peponi, C. Weder, E. J. Foster, J. M. Kenny and E. Gimenez, J. Polym. Sci., Part A: Polym. Chem., 2014, 52, 3123–3133 CrossRef CAS PubMed.
- R. Rusli and S. J. Eichhorn, Appl. Phys. Lett., 2008, 93, 033111 CrossRef PubMed.
- C. C. Sun, J. Pharm. Sci., 2005, 94, 2132–2134 CrossRef CAS PubMed.
- J. S. Haghpanah, R. Tu, S. Da Silva, D. Yan, S. Mueller, C. Weder, E. J. Foster, I. Sacui, J. W. Gilman and J. K. Montclare, Biomacromolecules, 2013, 14, 4360–4367 CrossRef CAS PubMed.
- M. Jorfi, M. N. Roberts, E. J. Foster and C. Weder, ACS Appl. Mater. Interfaces, 2013, 5, 1517–1526 CAS.
- S. Lee, A. Sonseca, R. Vadrucci, E. Gimenez, E. J. Foster and Y. Simon, J. Inorg. Organomet. Polym. Mater., 2014, 24, 898–903 CrossRef CAS PubMed.
- X. Dong, T. Kimura, J. Revol and D. Gray, Langmuir, 1996, 12, 2076–2082 CrossRef CAS.
- B. Braun and J. R. Dorgan, Biomacromolecules, 2009, 10, 334–341 CrossRef CAS PubMed.
- T. Kokubo, H. Kushitani, S. Sakka, T. Kitsugi and T. Yamamuro, J. Biomed. Mater. Res., 1990, 24, 721–734 CrossRef CAS PubMed.
- R. Maliger, P. J. Halley and J. J. Cooper-White, J. Appl. Polym. Sci., 2013, 127, 3980–3986 CrossRef CAS PubMed.
- Q. Chen, Biomedical Materials and Diagnostic Devices, ed. A. Tiwari, M. Ramalingam, H. Kobayashi and A. P. F. Turner, John Wiley & Sons, Inc., MA, USA, 2012, pp. 529–560 Search PubMed.
- A. Patel, A. K. Gaharwar, G. Iviglia, H. Zhang, S. Mukundan, S. M. Mihaila, D. Demarchi and A. Khademhosseini, Biomaterials, 2013, 34, 3970–3983 CrossRef CAS PubMed.
- A. Pei, J. Malho, J. Ruokolainen, Q. Zhou and L. A. Berglund, Macromolecules, 2011, 44, 4422–4427 CrossRef CAS.
- Y. Tien and K. Wei, Macromolecules, 2001, 34, 9045–9052 CrossRef CAS.
- Q. Wu, M. Henriksson, X. Liu and L. A. Berglund, Biomacromolecules, 2007, 8, 3687–3692 CrossRef CAS PubMed.
- G. Odian, Principles of Polymerization, John Wiley & Sons, Inc., New Jersey, 4th edn., 2004, pp. 39–197 Search PubMed.
- S. H. Pinner, J. Polym. Sci., 1956, 21, 153–157 CrossRef CAS PubMed.
- M. A. Hood, C. S. Gold, F. L. Beyer, J. M. Sands and C. Y. Li, Polymer, 2013, 54, 6510–6515 CrossRef CAS PubMed.
- A. K. Gaharwar, A. Patel, A. Dolatshahi-Pirouz, H. Zhang, K. Rangarajan, G. Iviglia, S. Shin, M. A. Hussain and A. Khademhosseini, Biomater. Sci., 2015, 3, 46–58 RSC.
- Y. Li and J. A. Ragauskas, Advances in diverse industrial applications of nanocomposites, ed. B. Reddy, InTech, 2011, pp. 18–36 Search PubMed.
- S. Liang, W. D. Cook and Q. Chen, Polym. Int., 2012, 61, 17–22 CrossRef CAS PubMed.
- B. Amsden, Soft Matter, 2007, 3, 1335–1348 RSC.
- Y. Chandorkar, G. Madras and B. Basu, J. Mater. Chem. B, 2013, 1, 865–875 RSC.
- S. Liang, X. Yang, X. Fang, W. D. Cook, G. A. Thouas and Q. Chen, Biomaterials, 2011, 32, 8486–8496 CrossRef CAS PubMed.
- M. Partini and R. Pantani, Polym. Degrad. Stab., 2007, 92, 1491–1497 CrossRef CAS PubMed.
- X. Gu, D. Raghavan, T. Nguyen, M. R. VanLandingham and D. Yebassa, Polym. Degrad. Stab., 2001, 74, 139–149 CrossRef CAS.
- M. J. Yaszemski, R. G. Payne, W. C. Hayes, R. Langer and A. G. Mikos, Biomaterials, 1996, 17, 2127–2130 CrossRef CAS.
- Z. Ge, J. C. H. GoH, L. Wang, E. P. S. Tan and E. H. Lee, J. Biomater. Sci., Polym. Ed., 2005, 16, 1179–1192 CrossRef CAS PubMed.
- M. Sabir, X. Xu and L. Li, J. Mater. Sci., 2009, 44, 5713–5724 CrossRef CAS.
- D. E. T. Shepherd and B. B. Seedhom, Rheumatology, 1999, 38, 124–132 CrossRef CAS PubMed.
- A. Thambyah, A. Nather and J. Goh, Osteoarthritis Cartilage, 2006, 14, 580–588 CrossRef CAS PubMed.
- J. Lee and D. Boughner, Circ. Res., 1985, 57, 475–481 CrossRef CAS.
- Q. Chen, S. Liang, J. Wang and G. P. Simon, J. Mech. Behav. Biomed. Mater., 2011, 4, 1805–1818 CrossRef CAS PubMed.
- T. Schepull, J. Kvist, C. Andersson and P. Aspenberg, BMC Musculoskeletal Disord., 2007, 8, 116 CrossRef PubMed.
- Q. Liu, J. Wu, T. Tan, L. Zhang, D. Chen and W. Tian, Polym. Degrad. Stab., 2009, 94, 1427–1435 CrossRef CAS PubMed.
- Q. Chen, L. Jin, W. D. Cook, D. Mohn, E. L. Lagerqvist, D. A. Elliott, J. M. Haynes, N. Boyd, W. J. Stark, C. W. Pouton, E. G. Stanley and A. G. Elefanty, Soft Matter, 2010, 6, 4715–4726 RSC.
- X. Yang, E. Bakaic, T. Hoare and E. D. Cranston, Biomacromolecules, 2013, 14, 4447–4455 CrossRef CAS PubMed.
- T. Kovacs, V. Naish, B. O'Connor, C. Blaise, F. Gagné, L. Hall, V. Trudeau and P. Martel, Nanotoxicology, 2010, 4, 255–270 CrossRef CAS PubMed.
- S. Dong, A. A. Hirani, K. R. Colacino, Y. W. Lee and M. Roman, Nano LIFE, 2012, 02, 1241006 CrossRef.
- M. J. D. Clift, E. J. Foster, D. Vanhecke, D. Studer, P. Wick, P. Gehr, B. Rothen-Rutishauser and C. Weder, Biomacromolecules, 2011, 12, 3666–3673 CrossRef CAS PubMed.
- M. M. Pereira, N. R. B. Raposo, R. Brayner, E. M. Teixeira, V. Oliveira, C. C. R. Quintão, L. S. A. Camargo, L. H. C. Mattoso and H. M. Brand, Nanotechnology, 2013, 24, 075103 CrossRef CAS PubMed.
- M. Jorfi and E. J. Foster, J. Appl. Polym. Sci., 2015, 132, 41719, DOI:10.1002/app.41719.
- K. A. Mahmoud, J. A. Mena, K. B. Male, S. Hrapovic, A. Kamen and J. H. T. Luong, ACS Appl. Mater. Interfaces, 2010, 2, 2924–2932 CAS.
- B. Jia, Y. Li, B. Yang, D. Xiao, S. Zhang, A. V. Rajulu, T. Kondo, L. Zhang and J. Zhou, Cellulose, 2013, 20, 1911–1923 CrossRef CAS.
- J. M. Dugan, J. E. Gough and S. J. Eichhorn, Biomacromolecules, 2010, 11, 2498–2504 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: CNC properties, 1H-NMR, IR and DSC complementary studies. See DOI: 10.1039/c5ra06768e |
| This journal is © The Royal Society of Chemistry 2015 |




![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O region (1800–1600 cm−1). Thick lines in the graphs correspond to low crosslinking degree samples, and thin lines correspond to high crosslinking degree samples. Top, for 1
O region (1800–1600 cm−1). Thick lines in the graphs correspond to low crosslinking degree samples, and thin lines correspond to high crosslinking degree samples. Top, for 1


