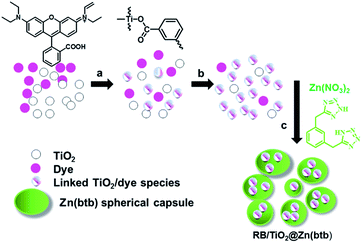
Tetrazole-based infinite coordination polymer for encapsulation of TiO2 and its potential application for fabrication of ZnO@TiO2 core–shell structures†
Sedigheh Abedi and
Ali Morsali*
Department of Chemistry, Faculty of Sciences, Tarbiat Modares University, P.O. Box 14115-4838, Tehran, Iran. E-mail: morsali_a@modares.ac.ir; Fax: +98 21 8009730; Tel: +98 21 82884416
First published on 2nd June 2015
Abstract
A new and facile technique for the preparation of ZnO@TiO2 core–shell nano/micro sphere structures from tetrazolate ligand, (1,3-bis(tetrazol-5-ylmethyl)benzene (btb)), was addressed. Via a solvent-induced precipitation method, nanoparticles of TiO2 bearing Rhodamine B were encapsulated into the Zn(btb) hollow spheres. The resulting polymeric carriers were characterized by SEM, HRTEM, EDS and XRD analyses and subsequently were used as precursors for the preparation of crystalline zinc oxide mineral spheres containing TiO2 species using a calcination route.
Introduction
Molecular encapsulation is an attractive supramolecular strategy because it is inherently flexible and does not necessarily require time-consuming synthetic processes. Indeed, molecular encapsulation is an effective way to recycle familiar dyes that are already well-characterized.1Two general supramolecular encapsulation strategies, including robust molecular containers and soft assemblies have been areas of rapid growth.2 Coordination compounds with infinite structures have been intensively studied, in particular, compounds with backbones constructed from metal ions as connectors and ligands as linkers, so-called coordination polymers. The phrase, “coordination polymers” appeared in the early 1960s, and the area was first reviewed in 1964.3
Versatile synthetic approaches for the assembly of target structures from molecular building blocks have been developed. The key to success is the design of the molecular building blocks, which directs the formation of the desired architectural, chemical, and physical properties of the resulting solid-state materials. In a surprisingly short time, the structural chemistry has attained a very mature level.2
Recently, micro- and nanoscale amorphous infinite coordination polymers (ICPs) as a subclasses of three generation dimension-based categories of porous coordination compounds,2 have attracted great attentions because of their promising useful chemical and physical properties and potential applications as asymmetric catalysis, mixture separations, gas storage, and biosensing.4–6 Straightforward fabrication of cross linked submicrometer functional metal–organic spheres, via polymerization and fast precipitation with a poor solvent, has been progressed by Oh, Mirkin, Wang and co-workers.4–6 Maspoch and co-workers7 developed a new and versatile method based on the polymerization following a precipitation fabrication process, leading to metal–organic polymeric micro- and nanometer-sized spheres as novel functional encapsulating matrices. Metal–organic hollow spheres by infinite coordination polymerization of ZnII metal ions and 1,4-bis(imidazol-1-ylmethyl)benzene (bix) have accordingly been synthesized. They showed that these hollow spheres are capable to surround the functional species present in the reaction mixture; leading to formation of encapsulated the desired species. Very recently, we could successfully introduced new metal–organic capsules, Zn(btb), prepared by metal node (Zn2+) and a new multidentate organic ligand, 1,3-bis(tetrazol-5-ylmethyl) benzene (btb) ligand. In addition, the potential application of the polymeric carriers was demonstrated in encapsulation of multifunctional guests, comprising quantum dots (QDs), ferromagnetic nanoparticles of iron oxide (Fe3O4), and dyes (fluorescein isothiocyanate (FITC), and Rhodamine B). Calcification of the prepared final ICPs exploited crystalline zinc oxide mineral spheres, rods and coral shapes.8
We decided to examine the encapsulation talent of tetrazolate ICPs hollow spheres for entrapment of TiO2 species, expecting to preparation of a new type of ZnO@TiO2 core–shell structures.9 Over the past few decades, some investigations have been carried out for design and preparation of TiO2 and ZnO based nanocomposites.10 Besides the use of TiO2 as a dielectric layer, it has a wide range of applications such as gas sensors, photo and thermal catalysis, photoelectrocatalysis,11 due to its excellent chemical and photochemical stability and being capable of photo-oxidative destruction of most organic pollutants.12
TiO2 based nanocomposite thin films such as ZnO/TiO2, Fe2O3/ZnO/TiO2 and Fe2O3/TiO2 were developed by Yang et al.13 on titanium substrates by applying a dip-coating technique using the sol–gel method. Wang et al.14 prepared three-dimensional ZnO@TiO2 hierarchical structures via a combination of an electrospinning and a hydrothermal technique. By adjusting the experimental parameters, two different morphologies of ZnO@TiO2 heteroarchitectures were achieved. Zn-doped TiO2 nanofibers shelled with ZnO hierarchical nanoarchitectures have been fabricated by combining electrospinning of TiO2 nanofibers and metal–organic chemical vapor deposition (MOCVD) of ZnO.15 Uyar et al. have fabricated core–shell heterojunction (CSHJ) nanofibers from ZnO and TiO2 core–shell structures in two combinations via electrospinning and atomic layer deposition, respectively which were then subjected to calcination.16
Herein, we describe that typically solvent-induced precipitation method following by calcination process, revealed a new facile way for preparation of ZnO@TiO2 core–shell nano/micro sphere structures.
Experimental section
Synthesis of btb ligand
To a 250 mL round-bottomed flask was added the nitrile (20 mmol), sodium azide (60 mmol), zinc bromide (20 mmol), and 40 mL of water. The reaction mixture was refluxed for 48 h with vigorous stirring. After cooling to room temperature, HCl (3 N, 30 mL) were added, and vigorous stirring was continued until the aqueous layer had a pH of 1. Subsequently, 200 mL of 0.25 N NaOH was added, and the mixture stirred for 30 min, until the original precipitate was dissolved and a suspension of zinc hydroxide was formed. The suspension was filtered, and the solid washed with 20 mL of 1 N NaOH. 40 mL of 3 N HCl was added to the filtrate with vigorous stirring causing the tetrazole to precipitate. The tetrazole was filtered and washed with HCl and dried in a drying oven to furnish the tetrazole as a white or slightly colored powder. The product had the following data: mp 269–270 °C.1H NMR (d-DMSO): 7.27 (t, 1H), 7.25 (b, 1H), 7.24 (b, 1H), 7.17 (m, 3H), 4.2 (s, 4H).
13C NMR: 173, 155, 136.5, 129.16, 127.9, 29.60.
Anal. calcd for btb: C, 49.57; H, 4.13; N, 46.28. Found: C, 49.12; H, 4.04; N, 43.86.
Encapsulation of TiO2 linked RB in hollow Zn(btb) spheres (RB/TiO2@Zn(btb))
In a typical experiment, a mixture of btb (242 mg, 1 mmol) in DMF (40 mL), Rhodamine B (20 μL, 200 ppm) and TiO2 (1 mg, Degussa P25) was prepared. The mixture was homogenized by sonication and stirred for specific times (30 min to 12 h) at room temperature. After overnight stirring, a methanolic solution (10 mL) of Zn(NO3)2·6H2O (296 mg, 1 mmol) was added, to generate the nano-micro spheres encapsulate TiO2 containing-dye nanoparticles. The resulting encapsulated metal–organic systems in all these cases were purified by centrifugation and washed three times with DMF, and redispersed in DMF to obtain the corresponding colloidal solutions was named RB/TiO2@Zn(btb).IR for RB/TiO2@Zn(btb) (KBr pellet, cm−1): 596 (w), 698 (w), 761 (m), 1105 (m), 1182 (w), 1253 (w), 1432 (w), 1487 (s), 1655 (s) 2931 and 3405 (m).
Preparation of ZnO@TiO2 core–shell spheres
The RB/TiO2@Zn(btb) hallow spheres was placed in a furnace and calcined at three temperatures, 400, 500 and 550 °C for 3 h in air. After 3 h holding, the generated precipitates were cooled to room temperature.X-ray powder diffraction (XRD) measurements were performed using a X'pert diffractometer manufactured by Philips with monochromatized CuKα radiation. The samples were characterized with a scanning electron microscope (SEM) (Philips XL 30) with a gold coating. Transmission electron microscopy (TEM) images were obtained with a Hitachi H-9500 apparatus. NMR data were collected by a BRUKER DRX500 AVANCE. An ultrasonic bath (Tecna 6; 50–60 Hz and 0.138 kW) was used for the ultrasonic irradiation. PL spectra were measured by means of a spectrofluorimeter manufactured by Cary eclipse FL0912M014.
Results and discussion
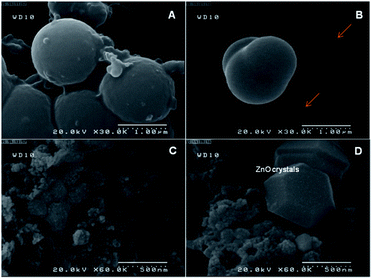
As described in experimental section, RB/TiO2@Zn(btb) hallow spheres were prepared by addition of a methanolic solution of Zn(NO3)2·6H2O to a TiO2 suspension containing a dye in DMF solvent. For more precise structural characterization of enclosing the TiO2 species into the Zn(btb) hollow spheres, ascribed to our previous report8 as well directing their multifunctional ability, RB was used in the mother reaction mixture. Strong interaction mode between the functional groups of dyes and the surface sites of TiO2 by a chemical bond lead to anchoring of dyes onto the TiO2 surface. Whereas, RB is known to be bound on the TiO2 surface via esterification between the carboxylic group of the dye and the surface hydroxyl of TiO2 (Scheme 1), it was also chosen for the mentioned monitoring purpose.17 Moreover, the dye encapsulation talent of this procedure, had been proved before.8Therefore, it was a commensurate examinee to contribute encapsulation process of TiO2 within the Zn(btb) spheres. Actually, it was found that in the presence of 3 mg of TiO2, i.e. more than optimized amount (1 mg), some uncaptured TiO2 species remain intact. SEM images of this sample represented in Fig. 1, clearly indicate the constructed coral shape capsules besides the unentrapped TiO2 particles. Furthermore, calcinations of the precipitate sample at 400 °C lead to formation of some segregated ZnO crystals and other aggregated TiO2 particles. During the optimization process of concentration and time, it was understood that in the presence of 1 mmol of tetrazolate ligand, 1 mmol of zinc salt, 1 mg of the commercial TiO2, and 20 μL of 200 ppm RB solution, best result of encapsulated TiO2 containing dye spheres were obtained. Whole the following evidences descried bellow let us to deduce the overall encapsulation process as demonstrated in Scheme 1. In fact, in the mixture containing suitable amount of the commercial TiO2 and in the presence of RB, the dye molecules are anchored to the titania surface. During overnight stirring, formation of these linked species becomes more completed. Once entrance of the btb ligand and the zinc salt, construction of the hallow polymeric structures of Zn(btb) is started and coincidently, encapsulation of the present titania bearing RB is occurred.
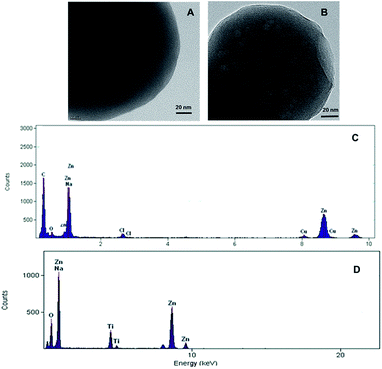
Fig. 2 shows FESEM images of the as-synthesized ICPs of these new particles. These images revealed the formation of spherical particles, RB/TiO2@Zn(btb), with approximate dimensions of 225 nm. In the first investigations, encapsulation of TiO2 species into the spheres of the metal–organic polymer of Zn(btb) was proved by the high-resolution transmission electron microscopy (HRTEM) images of the final precipitate. As it shows in Fig. 3 spherical particles with diameters in the range 100–2500 nm have been formed. Comparison of the infrared spectrum taken of the bare Zn(btb) and RB/TiO2@Zn(btb), not only showed that the ligand has been coordinated to metal ions, as evidenced by two strong peaks at 1655 and 1432 cm−1 represented the presence of deprotonated tetrazolate groups,18 but also indicated nearly identical intact patterns (Fig. S5 and S6 in ESI†). Moreover, upper scales of TEM images, clearly confirmed surrounding the TiO2 species with the Zn(btb) spherical polymers (Fig. 3b). Indeed, control experiments performed by adding a methanolic solution of TiO2 particles to a presynthesized colloidal solution of Zn(btb) spheres did not show encapsulation of this particles, while physisorption onto the external surface of the metal–organic spheres and some self titania aggregation occurred. Hence, we were encouraged to elucidate their character precisely with more characterization of these new coordination polymers.
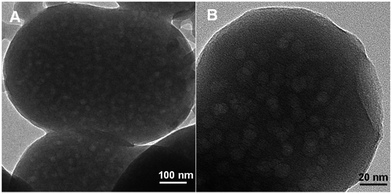 | ||
| Fig. 3 HRTEM images of RB/TiO2 encapsulated within the spherical Zn(btb) denoted as RB/TiO2@Zn(btb). | ||
Comparison of chemical composition of the Zn(btb) spheres with the RB/TiO2@Zn(btb) spheres, which was determined by and energy dispersive X-ray (RX-EDS) microanalysis (Fig. 4), right confirming HRTEM images (Fig. 4, left), showed that the later component contains titania species that do not exist in the bare spheres.
In addition, the recent reported multifunctional capability of these micro and nanometer size metal–organic capsules7,8 provides the other opportunity for them to be monitored via choosing an appropriate strategy. As, it was mentioned above, we applied RB, as a probe during the preparation step, not only to illustrate the multifunctional capability of this new carrier capsules, but also to arrange a way for distinguishing the resultant products. Fluorescence optical microscope images and the corresponding fluorescence spectrum of the bare Zn(btb) and encapsulated spheres of RB/TiO2@Zn(btb) have been presented in Fig. 5. These images evidently indicated the existence of the dye species within the capsules, which means that encapsulation process has performed successfully. In the previous work, we indicated the potential application of tetrazole-based infinite coordination polymers for fabrication of mineral zinc oxide nano and micro structures.8 Regarding to this capability of our simple method as well importance of zinc oxide as a versatile inorganic oxide in advanced materials and industrial chemicals, such as field effect transistors,19 solar cells,20 luminescent materials,21 photocatalysts,22 chemical sensors,23 construction of a new type of ZnO@TiO2 core–shell structures seemed to be achievable. Among various morphology of ZnO micro and nanostructures, those structures with hollow cavities have attracted a great deal of attention owing to their unique architecture such as low density, high surface area, good surface permeability,24,25 and broad applications in biomimetic studies, drug-delivery,26 catalysis, inks and pigments,27,28 chemical storage,29 microcapsule reactors,30 sensors, etc.31
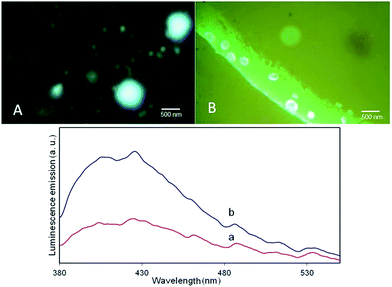 | ||
| Fig. 5 Representative fluorescence optical microscope images (up); and the fluorescence spectrum of (a) Zn(btb) and (b) RB/TiO2@Zn(btb). | ||
To clarify this aptitude, RB/TiO2@Zn(btb) hallow spheres was placed in a furnace and calcined at two temperatures, 400 and 500 °C for 3 h in air. As evidenced by SEM analysis, in both temperatures, spherical shape of spheres was retained. However, the original shapes of nano and micro spheres remain approximately intact after calcination at 500 °C. The adhesive spherical structures obtained at 400 °C, whereas separated spheres were prepared during calcination at 500 °C (Fig. 2c–f). Interestingly, calcination of the spheres at 550 °C led to stick some of them to the others (Fig. 2g). Although the reason of these observations was not determined yet, it may ascribe to different removal rate of the solvent from inside the spherical polymers at these two various temperatures. On the other hand, irradiation of the final RB/TiO2@Zn(btb) suspension calcined at 500 °C, in an ultrasonic bath (138 W, 30 min) made some broken spheres (Fig. 2). As evidenced by FESEM, represented in Fig. 2h, some microsize capsules have been exploded and their inside components obviously become clear. With more precise vision into the exploded formed spheres, not only the hollow space interior the ZnO spheres can be observed, but also surrounded TiO2 species with a shell of ZnO structure can be clearly monitored from the SEM image.
The powder XRD pattern recorded for the RB/TiO2@Zn(btb) hallow spheres and its calcination sample at 500 °C, denoted as RB/TiO2@Zn(btb)-500 in comparison with PXRD spectrum of ZnO and TiO2 (P25) are represented in Fig. 6. As might be expected, no distinguished diffraction peaks identify in XRD analysis of the RB/TiO2@Zn(btb) hallow spheres which is a sign of their amorphous character (Fig. 6a). After calcination process, PXRD pattern of RB/TiO2@Zn(btb)-500, contains both ZnO and TiO2 diffraction peaks. This observation, combined with EDS and SEM analyses, accordingly confirm TiO2 particles embedded into ZnO structure. The zinc containing polymeric capsules of RB/TiO2@Zn(btb) crystallize in the wurtzite ZnO structure which is the most stable phase in ambient conditions, and the titania phase PXRD pattern includes the anatase and rutile TiO2 phases that is identically recognized the Degussa P25 crystalline phase. Diffuse reflectance UV-vis spectroscopy of the bare Zn(btb), RB/TiO2@Zn(btb), Degussa P25 and ZnO represented in Fig. 7. As it shown, the red shift assigned in RB/TiO2@Zn(btb) spectrum obviously confirmed RB/TiO2 entrance into the Zn(btb) structure. Additionally, after calcination at 500 °C, while evaporation of whole organic content, the representative reflectance pattern of RB/TiO2@Zn(btb) completely alters and confirming the PXRD analysis, become resemble with crystalline ZnO structure (Figure dotted red and violet patterns). More efforts for characterization and finding the electronic potential of this kind of nanocomposites are currently in progress in our laboratory.
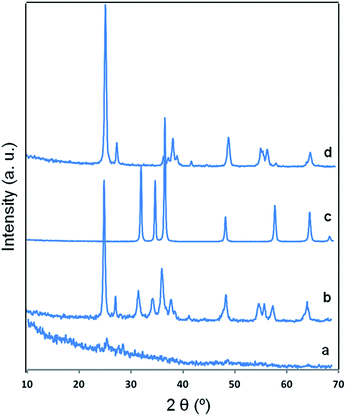 | ||
| Fig. 6 PXRD diffraction patterns of (a) RB/TiO2@Zn(btb), (b) calcined RB/TiO2@Zn(btb) at 500 °C, 3 h, (c) crystalline ZnO and (d) TiO2 Degussa P25. | ||
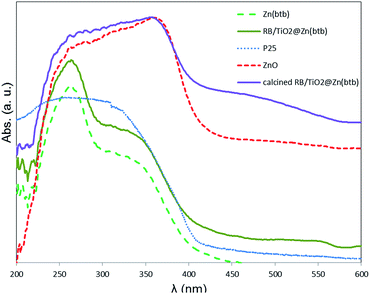 | ||
| Fig. 7 Comparison of the diffuse reflectance UV-vis spectroscopies of the bare Zn(btb) and RB/TiO2@Zn(btb), with Degussa P25 and ZnO as well calcinated RB/TiO2@Zn(btb) at 500 °C. | ||
Conclusions
In summary, we have demonstrated a new simple method for construction of micro/nano size spherical Zn(btb) polymers containing dye anchored TiO2 species. Encapsulation of the commercial Degussa P25 linked to the Rhodamine B, within the hallow space of Zn(btb) was accordingly investigated by SEM, HRTEM, XRD, EDS, Diffuse reflectance and Fluorescence spectroscopy. Subsequently, it has been indicated that using a solvent-induced precipitation method followed by a heating step, a type of TiO2@ZnO core–shell micro/nano spheres can be designed.Notes and references
- E. Arunkumar, C. C. Forbes and B. D. Smith, Eur. J. Org. Chem., 2005, 4051 CrossRef CAS PubMed.
- S. Kitagawa, R. Kitaura and S.-I. Noro, Angew. Chem., Int. Ed., 2004, 43, 2334 CrossRef CAS PubMed.
- J. C. Bailar Jr, Coordination Polymers, Prep. Inorg. React., 1964, 1 Search PubMed.
- (a) M. Oh and C. A. Mirkin, Nature, 2005, 438, 651 CrossRef CAS PubMed; (b) M. Oh and C. A. Mirkin, Angew. Chem., Int. Ed., 2006, 45, 5492 CrossRef CAS PubMed.
- Y.-M. Jeon, J. Heo and C. A. Mirkin, J. Am. Chem. Soc., 2007, 129, 7480 CrossRef CAS PubMed.
- (a) H. Maeda, M. Hasegawa, T. Hashimoto, T. Kakimoto, S. Nishio and T. Nakanishi, J. Am. Chem. Soc., 2006, 128, 10024 CrossRef CAS PubMed; (b) X. Sun, S. Dong and E. Wang, J. Am. Chem. Soc., 2005, 127, 13102 CrossRef CAS PubMed; (c) H. Wei, B. Li, Y. Du, S. Dong and E. Wang, Chem. Mater., 2007, 19, 2987 CrossRef CAS; (d) S. Jung and M. Oh, Angew. Chem., Int. Ed., 2008, 47, 2049 CrossRef CAS PubMed; (e) M. Y. Masoomi and A. Morsali, Coord. Chem. Rev., 2012, 256, 2921 CrossRef CAS PubMed; (f) L. Hashemi and A. Morsali, CrystEngComm, 2012, 14, 8349 RSC.
- I. Imaz, J. Hernando, D. Ruiz-Molina and D. Maspoch, Angew. Chem., Int. Ed., 2009, 48, 2325 CrossRef CAS PubMed.
- Z. Sharifzadeh, S. Abedi and A. Morsali, J. Mater. Chem. A, 2014, 2, 4803 CAS.
- S. Panigrahi and D. Basak, Nanoscale, 2011, 3, 2336 RSC.
- (a) N. Wang, X. Li, Y. Wang, Y. Hou, X. Zou and G. Chen, Mater. Lett., 2008, 62, 3691 CrossRef CAS PubMed; (b) N. Wang, C. Sun, Y. Zhao, S. Zhou, P. Chena and L. Jiang, J. Mater. Chem., 2008, 18, 3909 RSC; (c) M. Agrawal, S. Gupta, A. Pich, N. E. Zafeiropoulos and M. Stamm, Chem. Mater., 2009, 21, 5343 CrossRef CAS.
- (a) C. P. Chang, J. N. Chen, M. C. Lu and H. Y. Yang, Chemosphere, 2005, 58, 1071 CrossRef CAS PubMed; (b) J. Virkutyte, B. Baruwati and R. S. Varma, Nanoscale, 2010, 2, 1109 RSC; (c) P. Atienzar, T. Ishwara, M. Horie, J. R. Durrant and J. Nelson, J. Mater. Chem., 2009, 19, 5377 RSC; (d) M. Alvaro, E. Carbonell, P. Atienzar and H. Garcia, ChemPhysChem, 2006, 7, 1996 CrossRef CAS PubMed; (e) Y. Qu, H. Min, Y. Wei, F. Xiao, G. Shi, X. Li and L. Jin, Talanta, 2008, 76, 758 CrossRef CAS PubMed.
- (a) J. A. Navio, C. Cerrillos, M. A. Pradera, E. Morales and J. L. Gomez-Ariza, Langmuir, 1998, 14, 388 CrossRef CAS; (b) K. T. Ranjit and I. Willner, J. Phys. Chem. B, 1998, 102, 9397 CrossRef CAS; (c) C. Hea, B. Tiana and J. Zhang, J. Colloid Interface Sci., 2010, 344, 382 CrossRef PubMed.
- S. Yang, X. Quan, X. Li, Y. Liu, S. Chen and G. Chen, Phys. Chem. Chem. Phys., 2004, 6, 659 RSC.
- N. Wang, C. Sun, Y. Zhao, S. Zhou, P. Chena and L. Jiang, J. Mater. Chem., 2008, 18, 3909 RSC.
- M. E. Fragalà, I. Cacciotti, Y. Aleeva, R. L. Nigro, A. Bianco, G. Malandrino, C. Spinella, G. Pezzotti and G. Gusmano, CrystEngComm, 2010, 12, 3858 RSC.
- S. Vempati, F. K. Ozgit-Akgun, I. Donmez, N. Biyikli and T. Uyar, Nanoscale, 2014, 6, 5735 RSC.
- (a) A. Nawrocka and S. Krawczyk, J. Phys. Chem. C, 2008, 112, 10233 CrossRef CAS; (b) C. Chen, W. Ma and J. Zhao, Chem. Soc. Rev., 2010, 39, 4206 RSC.
- (a) M. Dinca, A. F. Yu and J. R. Long, J. Am. Chem. Soc., 2006, 128, 17153 CrossRef CAS; (b) S. Jeong, X. Song, S. Jeong, M. Oh, X. Liu, D. Kim, D. Moon and M. S. Lah, Inorg. Chem., 2011, 50, 12133 CrossRef CAS PubMed.
- W. I. Park, J. S. Kim, G. C. Yi, M. H. Bae and H. J. Lee, Appl. Phys. Lett., 2004, 85, 5052 CrossRef CAS PubMed.
- S. H. Ko, D. Lee, H. W. Kang, K. H. Nam, J. Y. Yeo, S. J. Hong, C. P. Grigoropoulos and H. J. Sung, Nano Lett., 2011, 11, 666 CrossRef CAS PubMed.
- J. Zhang, W. Y. Yu and L. D. Zhang, Phys. Lett. A, 2002, 299, 276 CrossRef CAS.
- G. Ramakrishna and H. N. Ghosh, Langmuir, 2003, 19, 3006 CrossRef CAS.
- N. F. Hamedani, A. R. Mahjoub, A. A. Khodadadi and Y. Mortazavi, Sens. Actuators, B, 2011, 156, 737 CrossRef CAS PubMed.
- J. Yu and X. Yu, Environ. Sci. Technol., 2008, 42, 4902 CrossRef CAS.
- G. Patrinoiu, M. Tudose, J. M. Calderon-Moreno, R. Birjega, P. Budrugeac, R. Ene and O. Carp, J. Solid State Chem., 2012, 186, 17 CrossRef CAS PubMed.
- K. An and T. Hyeon, Nano Today, 2009, 4, 359 CrossRef CAS PubMed.
- (a) D. M. Vriezema, M. C. Aragones, J. A. A. W. Elemans, J. J. L. M. Comelissen, A. E. Rowan and R. J. M. Nolte, Chem. Rev., 2005, 105, 1445 CrossRef CAS PubMed; (b) Y. Zhao and L. Jiang, Adv. Mater., 2009, 21, 3621 CrossRef CAS PubMed.
- (a) F. Caruso, R. A. Caruso and H. Möhwald, Science, 1998, 282, 1111 CrossRef CAS; (b) B. D. G. Geest, N. N. Sanders, G. B. Sukhorukov, J. Demeester and S. C. D. Smedt, Chem. Soc. Rev., 2007, 36, 636 RSC.
- F. Caruso, Chem.–Eur. J., 2000, 6, 413 CrossRef CAS.
- X. W. Lou, L. A. Archer and Z. C. Yang, Adv. Mater., 2008, 20, 3987 CrossRef CAS PubMed.
- L. L. Wang, Z. Lou, T. Fei and T. Zhang, J. Mater. Chem., 2012, 22, 4767 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details anf IR spectroscopy of btb, Zn(btb) and RB/TiO2@Zn(btb) compounds as well more HRTEM images of Zn(btb) and RB/TiO2@Zn(btb), and EDX analysis (of SEM images) of RB/TiO2@Zn(btb), and calcined RB/TiO2@Zn(btb) spheres at 400 °C, 3 h are available. See DOI: 10.1039/c5ra06093a |
| This journal is © The Royal Society of Chemistry 2015 |