Investigation into the temperature sensing behavior of Yb3+ sensitized Er3+ doped Y2O3, YAG and LaAlO3 phosphors†
Guofeng Liua,
Linlin Fua,
Zhiyi Gaoa,
Xingxing Yanga,
Zuoling Fu*ab,
Zhiying Wangd and
Yanmin Yang*c
aKey Laboratory of Coherent Light, Atomic and Molecular Spectroscopy, Ministry of Education, College of Physics, Jilin University, Changchun 130023, China. E-mail: zlfu@jlu.edu.cn; Fax: +86-431-85167966; Tel: +86-431-85167966
bState Key Laboratory of Superhard Materials, Jilin University, Changchun 130012, China. E-mail: zlfu@jlu.edu.cn; Fax: +86-431-85167966; Tel: +86-431-85167966
cLuminescence and Display Research Institute, College of Physics Science and Technology, Hebei University, Baoding 071002, China. E-mail: mihuyym@163.com; Fax: +86-312-5079423; Tel: +86-312-5079423
dCollege of Chemistry and Biology, Beihua University, Jilin 132013, China
First published on 22nd May 2015
Abstract
The optical temperature sensors based on Y2O3 (YAG/LaAlO3):Yb3+/Er3+ have been successfully prepared by the sol–gel method. The sensing behavior of samples was studied as a function of temperature in the range 298–573 K for optical temperature measurement. The maximum sensor sensitivity derived from the ratio of fluorescence intensity (R) of the green upconversion (UC) emissions was approximately 0.0050 K−1 (Y2O3). The influence of the different structures and bonding of the hosts on the temperature sensitivities of Er3+/Yb3+ co-doped Y2O3, YAG and LaAlO3 was investigated in detail based on chemical bond theory. The calculated results could provide certain guiding significance for other relevant temperature sensing materials.
1. Introduction
Almost all physical, chemical and biological processes are temperature dependent, making accurate temperature knowledge essential for their control and understanding.1,2 There are many areas of industry where temperature measurements are essential, such as metallurgy, glass manufacturing, material modeling as well as food manipulation and testing.3 In most of these applications, contact-based temperature measurements (using thermocouples, thermistors or resistance thermometers) are not applicable. A fluorescent sensor that makes use of temperature-dependent fluorescence properties from a luminescent material can overcome the interference of strong electromagnetic noise, hazardous sparks, and a corrosive environment, which are inaccessible to traditional temperature-measurement methods.4 In this vast field, fluorescence thermometry appears as a tool for non-contact and non-invasive surface temperature measurements, and offers a number of advantages over conventional test methods (infrared pyrometry, thermocouples, thermistors). These include high accuracy, remote detection, high signal yield, cost-effectiveness, and quantitative global temperature/heating information.5 As two models of this technique, both the single-color fluorescence thermometry method and the two-color fluorescence thermometry technique are based on a thermophosphor whose fluorescence intensity is temperature dependent.6 Most luminescence-based thermometry mainly relies on the temperature-dependent fluorescence intensity of one transition, the accuracy of which is susceptible to errors introduced by optical occlusion, concentration inhomogeneities, excitation power fluctuations, or environment-induced non-radiative relaxation. Ratiometric detection, based on the intensity ratio of two independent emissions of the same phosphor, can circumvent these complications and give rise to more accurate self-referencing thermal sensing, and is thus gaining popularity.7Yb3+/Er3+ co-doped UC fluorescence materials are of interest for optical temperature sensors. In this method, the temperature of a sample can be obtained by measuring the signal ratio of two fluorescence lines associated with the excited states of trivalent rare earth ions, for example the two energy levels 2H11/2 and 4S3/2 in the Er3+ ion could be used. It is important to choose an appropriate material that presents a highly efficient fluorescence signal and a good thermal stability for successful practical applications of the fluorescence intensity ratio method. In addition, when the phonon energy of the matrix in which the rare earth ions are embedded is low, the intensity of the UC emission will be strong. However, NaYF4 has a low phonon energy, but its application is greatly restricted because of the poor chemical stability and low laser induced damage threshold of the hosts.8–10 Therefore it has been recognized that the host matrix plays an important role in the fluorescence properties of the rare earth-doped material. Y2O3, Y3Al5O12 (YAG) and LaAlO3 have better stable chemical properties for practical applications than traditional fluoride materials,11–15 which make Yb3+/Er3+ co-doped Y2O3, Y3Al5O12 (YAG) and LaAlO3 phosphors promising materials in optical temperature sensing. In addition, UC materials exhibit anti-Stokes emission upon low levels of irradiation in the near-infrared (NIR) spectral region with a sharp emission bandwidth, long lifetime, tunable emission, high photostability, and low cytotoxicity, which render them particularly useful for bio-imaging applications.16 In recent years, photothermal ablation (PTA) therapy has attracted much interest as a minimally invasive alternative to conventional approaches, such as surgery and chemotherapy, for therapeutic intervention at specific biological targets. In particular, NIR (λ = 700–1100 nm) laser induced PTA, which converts NIR optical energy into thermal energy, has gained increasing attention because the NIR laser is absorbed less by biological tissues, and the typical penetration depth of the NIR (such as 980 nm) light can be several centimeters in biological tissues. Therefore, Yb3+/Er3+ co-doped oxide phosphors could realize three functions: vivo imaging, temperature sensing, and PTA of cancer cells.17,18
Throughout the literature, we found that the sensitivities of different host materials based on the fluorescence intensity ratio (R) of Er3+ and Yb3+ were different. For example, the sensitivities in Al2O3 nanoparticles, La2O3 phosphor, LiNbO3 particles and phosphate glass ceramics were 0.0051 K−1, 0.0091 K−1, 0.0016 K−1 and 0.0075 K−1, respectively.12,19–21 Thus, it is necessary to understand which kind of material has higher sensitivity with high fluorescence efficiency and what the influencing factors are for the sensitivity of temperature sensing. In the present paper, we report the temperature sensing characteristics of Er3+/Yb3+ co-doped Y2O3, YAG and LaAlO3 powders. Then the factors affecting the sensitivity of temperature sensing are given and discussed in detail according to the experimental and theoretically calculated results.
2. Experimental section
2.1. Synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. Highly transparent solutions were obtained after stirring for a few minutes. Then the resultant mixtures were dried at 90 °C for 24 h to obtain light-brown dried gels. After grinding in an agate mortar, the gels were fired in air at 1200 °C for 4 h to get the corresponding phosphors.
2. Highly transparent solutions were obtained after stirring for a few minutes. Then the resultant mixtures were dried at 90 °C for 24 h to obtain light-brown dried gels. After grinding in an agate mortar, the gels were fired in air at 1200 °C for 4 h to get the corresponding phosphors.2.2. Characterization
The crystal structures were obtained from powder X-ray diffraction (XRD) measurements performed on a Rigaku-Dmax 2500 diffractometer at a scanning rate of 15° min−1 in the 2θ range from 10° to 80°, with graphite monochromatized Cu Kα radiation (λ = 0.15405 nm). The morphology and size of the obtained samples were examined by a field emission-scanning electron microscope (FE-SEM, XL30, Philips). All the measurements were performed at room temperature. The UC luminescence spectra were measured by an Andor Shamrock SR-750 fluorescence spectrometer. A CCD detector combined with a monochromator was used for signal collection from 400 to 750 nm. A diode laser tuned to 980 nm was used as the pump source. The diode was coupled to a fiber (core diameter 200 mm, numerical aperture 0.22). The luminescence spectra were recorded by exciting the samples with the 980 nm LD using an Andor SR-500i spectrometer (Andor Technology Co, Belfast, UK). The Yb3+/Er3+ co-doped Y2O3, YAG and LaAlO3 samples were filled in an iron sample cell and the temperature of the sample was increased from 298 K to 573 K by heating with resistive wire elements. A copper-constant thermocouple buried in the sample was used to monitor the sample's temperature with a measurement error of ±1.5 K.3. Results and discussion
3.1. Structural investigations
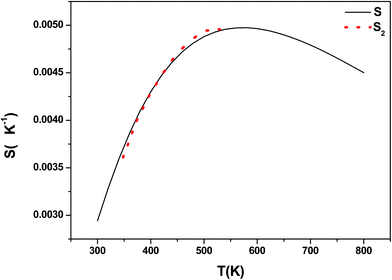
Fig. 1 shows the X-ray diffraction patterns of the Yb3+/Er3+ co-doped Y2O3, YAG and LaAlO3 phosphors. The results indicated that all diffraction peaks of Y2O3 phosphor could be readily indexed to the cubic phase of Y2O3 (JCPDS no. 41-1105), and the sample was of good purity. The XRD patterns of YAG phosphor were basically consistent with those of the cubic phase of YAG (JCPDS no. 09-0310), and no other impurities were observed. In contrast to the standard card (JCPDS no. 31-0022), the X-ray diffraction patterns of LaAlO3 phosphor indicated that LaAlO3 phosphor exhibited a pure hexagonal phase. Insets show the FE-SEM images of the Yb3+/Er3+ co-doped Y2O3, YAG and LaAlO3 phosphors, respectively. All phosphors were microcrystalline with flaky morphology.3.2. UC luminescence
We chose Y2O3 to investigate relative properties among Yb3+/Er3+ co-doped Y2O3, YAG and LaAlO3 phosphors. Fig. 2 presents the UC emission spectra of the Yb3+/Er3+ co-doped Y2O3 samples with different dopant concentrations under the excitation of a 980 nm diode laser. The results indicated that the UC emission intensity and spectral properties depended on the dopant concentrations. The spectra exhibited two distinct emission bands corresponding to green emission and red emission of Er3+ ions. Emissions were centered at 522, 563 and 660 nm, corresponding to green emissions from 2H11/2 and 4S3/2 and red emission from 4F9/2 excited states to the 4I15/2 ground state of Er3+ ions, respectively. When keeping the Er3+ ion concentration fixed at 0.01, and decreasing the concentration of Yb3+ (from 0.05 to 0.01), the intensity of green UC emission increased apparently with the red emission decreasing. This phenomenon can be explained by the following: in Yb3+/Er3+ co-doped materials, the energy transfer (ET), as a dominant mechanism, is due to the large absorption cross section of Yb3+ and Er3+ ions. When the concentration of Yb3+ decreased, the co-doping of Yb3+ greatly reduced and ET between Yb3+ and Er3+ greatly reduced, which decreased the population of the 4F9/2 level and relatively increased the population of 2H11/2 and 4S3/2 levels. As a result, the 4F9/2–4I15/2 transitions of Er3+ were considerably decelerated, resulting in the lessening of red emission in the Er3+/Yb3+ co-doped Y2O3 phosphor.22 The insets show the UC emission images of the five samples of Y2O3:xEr3+, yYb3+ (x = 0.01; y = 0.01, 0.02, 0.03, 0.04, 0.05) under 980 nm excitation. In particular, the green emission was strong enough to be seen by the naked eye in the Y2O3:0.01Er3+, 0.01Yb3+ sample. In addition, the UC emission spectra of the Yb3+/Er3+ co-doped YAG and LaAlO3 samples with different dopant concentrations under the excitation of the 980 nm diode laser were provided in Fig. S1 and S2 (ESI),† respectively. The spectra exhibited two distinct emission bands, corresponding to green emissions from 2H11/2 and 4S3/2 and red emission from 4F9/2 excited states to the 4I15/2 ground state of Er3+ ions and the UC emission intensities of LaAlO3 (0.01Er3+, 0.03Yb3+) and YAG (0.01Er3+, 0.05Yb3+) were relatively stronger than those of the other samples.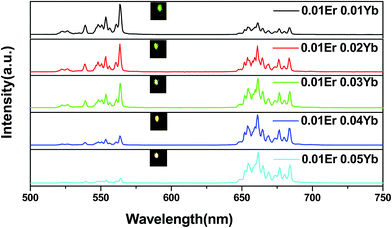 | ||
| Fig. 2 The UC luminescence spectra of Y2O3:xEr3+, yYb3+ (x = 0.01; y = 0.01, 0.02, 0.03, 0.04, 0.05). The insets show the UC emission images of the five samples under 980 nm excitation. | ||
Fig. 3 reveals the dependence of the intensity of UC peaks on the excitation power of the 980 nm diode laser. The intensity of UC luminescence increased remarkably with the increase of excitation power. The visible luminescence emission intensity of the UC phosphor and the infrared excitation power obey the relation Iμp ∝ (Ppump)n, where n is the number of pump photons used to populate the UC emission state and Iμp and Ppump represent the UC emission intensity and power, respectively.23–25 It can be observed that the slopes of the three fitted lines corresponding to 522, 563 and 660 nm emissions are 2.48, 2.34 and 1.84, respectively. The results indicated that two IR excitation photons were required to produce one UC photon in the system of Y2O3:0.01Yb3+, 0.01Er3+.23,26 At the same time, we obtained the values of YAG (0.01Er3+, 0.05Yb3+) and LaAlO3 (0.01Er3+, 0.03Yb3+). The slopes of the three fitted lines of YAG corresponding to 523, 559 and 669 nm emissions are 1.90, 2.17 and 1.76, respectively (Fig. S3, ESI†). For LaAlO3, the slopes of the three fitted lines corresponding to 520, 540 and 651 nm emissions are 1.86, 1.75, and 1.65 (Fig. S4, ESI†), respectively. The results indicated that two IR excitation photons were required to produce one UC photon in the systems of Y2O3, YAG and LaAlO3.27,28
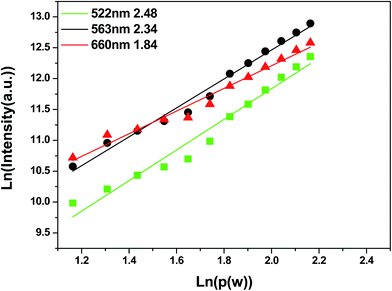 | ||
| Fig. 3 The log–log plots of UC emission intensity of Y2O3:0.01Yb3+, 0.01Er3+ as a function of the 980 nm laser pumping power. | ||
It is well known that there are two main emission bands corresponding to green emission and red emission, respectively, for Er3+ ions. The mechanism of UC luminescence in Er3+-doped materials has been extensively studied.27–30 A possible UC mechanism was proposed and is shown in Fig. 4. The Yb3+ ions at ground state can be excited to 2F5/2 (2F7/2 → 2F5/2) through a ground absorption (GSA) process. Then the metastable energy level 4I11/2 of Er3+ ions is populated by a first Yb3+ → Er3+ energy transfer (ET) step: 2F5/2 (Yb3+) + 4I15/2 (Er3+) → 2F7/2 (Yb3+) + 4I11/2 (Er3+); Er3+ in the 4I11/2 state is next excited to the 4F7/2 state by another ET: 2F5/2 (Yb3+) + 4I11/2 (Er3+) → 2F7/2 (Yb3+) + 4F7/2 (Er3+) or the excited state absorption (ESA): 4I11/2 (Er3+) + one photon → 4F7/2 (Er3+). Then multiphonon relaxation (MPR) happens and populates the lower levels 2H11/2 (4S3/2). After the first-level excitation, the same laser pumps the excited Er3+ from the 4I11/2 and 4I13/2 levels to the 4F7/2 and 4F9/2 levels, respectively. Subsequent MPR processes populate the 2H11/2 (4S3/2). The radiative relaxation from the thermally coupled levels 2H11/2 (4S3/2) and the 4F9/2 level to the ground state 4I15/2 of Er3+ results in the green emissions and red emissions, respectively.22,31
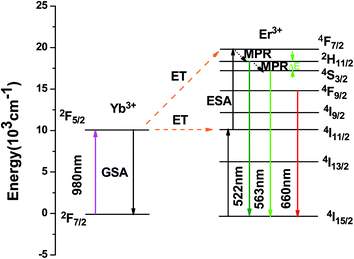 | ||
| Fig. 4 The energy level diagrams of Yb3+/Er3+ co-doped Y2O3 under excitation of 980 nm, and possible UC processes. | ||
3.3. Temperature sensing behavior
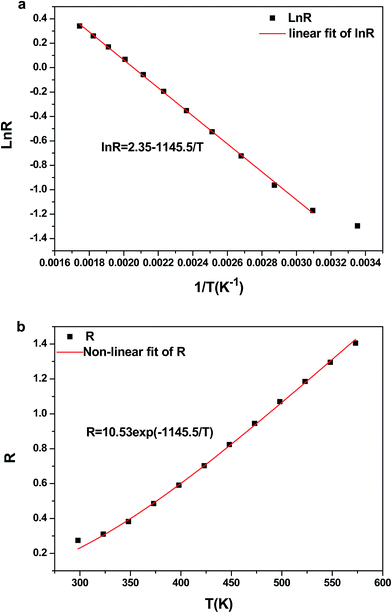
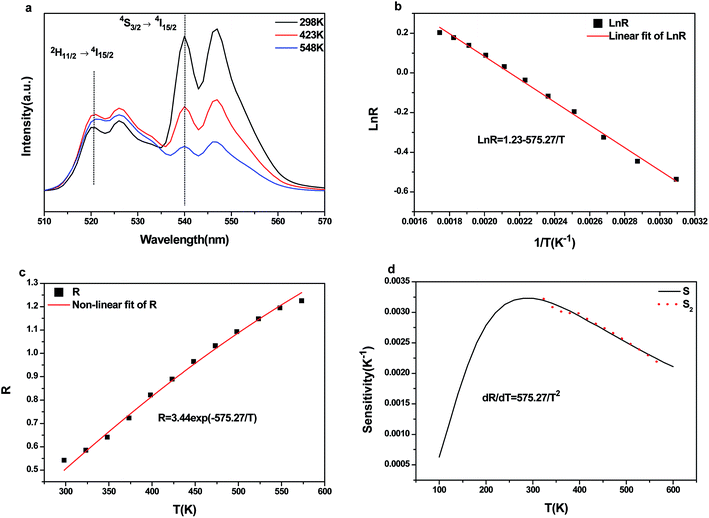
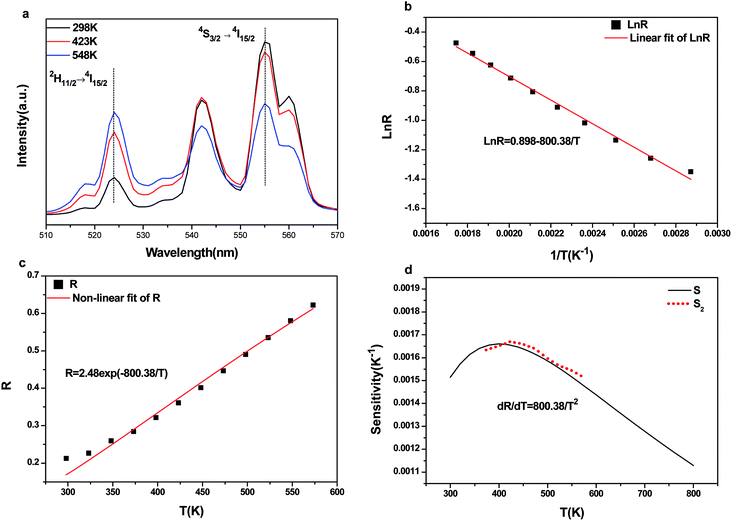
The temperature-dependent UC photoluminescent properties of Y2O3:Er3+, Yb3+ phosphor were investigated in terms of fluorescence intensities from two closely spaced energy levels (thermally coupled levels) in order to establish its potential as an optical temperature sensor. It has been shown that the relative population of such thermally coupled levels follows a Boltzmann type population distribution.31,32 Fig. 5 represents the UC emission spectra of Y2O3:0.01Er3+, 0.01Yb3+ phosphor from 298 K to 573 K; the pumping power of the 980 nm diode laser was set at 1.3 W. It was interesting to find that the fluorescence intensity ratio of the two emissions from 2H11/2/4S3/2 → 4I15/2 transitions increased with increasing temperature. This is because the state of 2H11/2 can be populated effectively from 4S3/2 by a thermalization process, which will lead to the relative variation of intensity between the two thermally coupled levels to ensure a quasi-thermal equilibrium according to the Boltzmann distribution. Fig. 8(a) and 9(a) show the green UC emission spectra of LaAlO3 (0.01Er3+, 0.03Yb3+) and YAG (0.01Er3+, 0.05Yb3+) at 298 K, 423 K and 548 K, respectively. It also can be observed that the fluorescence intensity ratio of the two emissions from 2H11/2/4S3/2 → 4I15/2 transitions increased with increasing temperature. Since the emission intensities are proportional to the population of each energy level, the ratio of fluorescence intensity from two thermally coupled energy levels can be written as follows.33–35
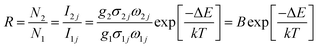 | (1) |
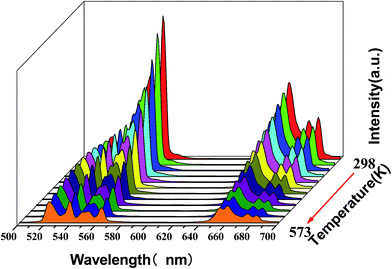 | ||
| Fig. 5 Temperature evolution of the Er3+ UC emission spectra excited by a 980 nm laser for the Y2O3:0.01Er3+, 0.01Yb3+ phosphor from 298 K to 573 K. | ||
N, g, σ, ω are the number of ions, the degeneracy, the emission cross-section and the angular frequency of fluorescence transitions from the upper (2H11/2) and lower (4S3/2) thermalizing energy levels to level 4I15/2, respectively. I2j and I1j are the integrated intensities of the 2H11/2 → 4I15/2 and 4S3/2 → 4I15/2 transitions, respectively. ΔE is the energy difference between the two thermally linked levels, kB is the Boltzmann constant and T is the absolute temperature.34 Fig. 6(a) shows the fluorescence intensity (integrated area below the fluorescence curves) ratio of green UC emissions I522 and I563 of the Y2O3 phosphor as a function of inverse absolute temperature in the range of 298–573 K on a monolog scale. The experimental data were fitted to a straight line with a slope of about 1145.5, which represents the value of ΔE/kB. The R of green UC emissions for the 2H11/2/4S3/2 → 4I15/2 transitions relative to temperature in the range of 298–573 K is presented in Fig. 6(b). We can observe that the value of R increases with the sample's temperature. The experimental data are fitted to eqn (1), which matches well, and the value of coefficient B is found to be 10.53. Fig. 8(b) and 9(b) show the fluorescence intensity ratio of green UC emission of LaAlO3 (0.01Er3+, 0.03Yb3+) and YAG (0.01Er3+, 0.05Yb3+) as a function of inverse absolute temperature in the range of 298–573 K on a monolog scale. The values of ΔE/kB are 575.27 and 800.38, respectively. Fig. 8(c) and 9(c) show the dependence of R for LaAlO3 (0.01Er3+, 0.03Yb3+) and YAG (0.01Er3+, 0.05Yb3+) on the temperature in the range of 298–573 K. The values of B for the best fit curves of the experimental data are found to be 3.44 and 2.48, for LaAlO3 (0.01Er3+, 0.03Yb3+) and YAG (0.01Er3+, 0.05Yb3+), respectively.
It is important to know the rate at which the fluorescence intensity ratio changes for a given change in temperature for sensing applications. The temperature sensitivity S can be defined as follows:36–38
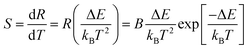 | (2) |
Eqn (2) suggests that using pairs of energy levels with larger energy differences increases the sensitivity of the fluorescence intensity ratio. However special attention must be given to ensuring that the levels are not too far apart to prevent thermalization from being observed. The sensitivity of Y2O3:Yb3+/Er3+ as a function of temperature is shown in Fig. 7. It can be observed that the sensitivity of Y2O3:0.01Yb3+, 0.01Er3+ reached the maximum of about 0.0050 K−1 at the temperature of 563 K. It can be seen from Fig. 8(d) and 9(d) that the sensitivity of LaAlO3 (0.01Er3+, 0.03Yb3+) and YAG (0.01Er3+, 0.05Yb3+) reached maxima of about 0.0032 K−1 at the temperature of 281 K and 0.0017 K−1 at the temperature of 404 K, respectively. In addition, relative parameters for Er3+ and Yb3+/Er3+ co-doped materials are shown in Table 1. It can be seen that the sensitivity alters from 0.0016 K−1 to 0.0091 K−1, as determined by B and ΔE/kB.
 | ||
| Fig. 7 The sensor sensitivity S = dR/dT as a function of temperature for the Y2O3:Yb3+/Er3+ phosphor. S2 represents the value of experimental sensitivity. | ||
| Host | Temperature range [K] | Excitation wavelength (nm) | Maximum sensitivity [K−1] | Ref. |
|---|---|---|---|---|
| Fluorotellurite glass | 300–550 | 800 | 0.0054 | 42 |
| Oxy-fluoride ceramics glass | 303–823 | 980 | 0.0047 | 43 |
| La2O2S phosphor | 290–573 | 971 | 0.0080 | 30 |
| LiNbO3 particles | 285–453 | 980 | 0.0075 | 21 |
| CaLa2ZnO5 phosphor | 298–513 | 980 | 0.0059 | 44 |
| Ga2S3:La2O3 glass | 293–498 | 1060 | 0.0052 | 45 |
| Al2O3 nanoparticles | 295–973 | 978 | 0.0051 | 12 |
| Er–Mo:Yb2Ti2O7 nanophosphor | 290–610 | 976 | 0.0074 | 38 |
| Er–Mo:Yb3Al5O12 nanocrystals | 295–973 | 980 | 0.0048 | 46 |
| GaWO4 phosphor | 303–873 | 980 | 0.0073 | 47 |
| Yttrium silicate powders | 300–600 | 975 (pulsed) | 0.0070 | 48 |
| Yttrium silicate powders | 300–600 | 975 | 0.0056 | 49 |
| Silicate glass | 296–723 | 978 | 0.0033 | 13 |
| Silicate glass | 296–673 | 978 | 0.0023 | 50 |
| Phosphate glass ceramics | 298–450 | 975 | 0.0016 | 20 |
| La2O3 phosphor | 303–600 | 980 | 0.0091 | 19 |
| Y2O3 | 298–573 | 980 | 0.0050 | This work |
| YAG | 298–573 | 980 | 0.0017 | This work |
| LaAlO3 | 298–573 | 980 | 0.0032 | This work |
Many host materials co-doped with Yb3+/Er3+ have been studied. It can be seen that ΔE differs little among different host materials. The shield of the outermost electrons 5s25p6 keeps the levels of trivalent rare earth ions from the influence of the crystal field of the hosts. Generally, the energy gap ΔE is decided by the even-parity of the crystal field of the hosts and is related to the symmetries of the crystal system. Then the S value is mainly determined by B, which can be rewritten as follows:30
It can be observed that B is determined only by Ωλ = 2, 4, 6.30 The crystal parameter Ωλ represents several properties of materials. The Ω6 parameter is related to the rigidity of the medium in which the ions are situated. The Ω2 is related to symmetry. It is sensitive to changes in constituents of materials, because these lead to changes in crystal structures, bond lengths, covalency and nephelauxetic effects. It is more sensitive to the environments than others among J–O parameters. Thus B is mainly determined by Ω2 and the Ω2 parameter is strongly affected by covalent chemical bonding.39 This means that sensitivity is associated with covalent chemical bonding. The covalency between O2− and Er3+ is different in the three samples.40 Since the Er3+ ions occupy the sites of Y and La ions, we calculated bond lengths and bond covalency of Y–O and La–O according to chemical bond theory.41 The calculated mean values of bond lengths and bond covalency are listed in Table 2. In addition, the related parameters for sensitivity of the different host materials (Y2O3, YAG and LaAlO3) are shown in Table 2. It can be observed that the values of B and S increase with increasing average bond covalency. This means that the sensitivity is proportional to the bond covalency. These experimental and theoretical results are important in increasing our understanding of the temperature-dependent luminescence of doped rare earth ions and in developing their future applications.
4. Conclusions
In conclusion, Yb3+ sensitized Er3+ doped Y2O3, YAG and LaAlO3 phosphors have been successfully prepared by the sol–gel method. We studied the temperature sensing behavior of samples and obtained the ratio of fluorescence intensity from two thermally coupled energy levels, which increased with increasing temperature. In addition, we quantified the sensitivity of the samples. The maxima of sensitivity of Y2O3, YAG and LaAlO3 were 0.0050 K−1, 0.0017 K−1, and 0.0032 K−1, respectively. We found that the sensitivity was strongly affected by bond covalency. Correspondingly, we calculated bond lengths and bond co-valencies of Y–O and La–O according to chemical bond theory. We found that the values of B and S increased with increasing average bond covalency, and that the calculated values were basically in good agreement with our experimental results. The experimental and theoretical results are important in increasing our understanding of the temperature-dependent luminescence of doped rare earth ions and in developing their future applications.Acknowledgements
This work was supported by the Science and Technology Development Planning Project of Jilin Province (20130522173JH), partially sponsored by the China Postdoctoral Science Foundation, supported by the National Foundation for Fostering Talents of Basic Science (no. J1103202) and by the Outstanding Young Teacher Cultivation Plan in Jilin University.References
- N. Rakov and G. S. Maciel, Dalton Trans., 2014, 16025–16030 RSC.
- X. Wang, X. G. Kong, Y. Yu, Y. J. Sun and H. Zhang, J. Phys. Chem. C, 2007, 111, 15119–15124 CAS.
- B. P. Singh, A. K. Parchur, R. S. Ningthoujam, P. V. Ramakrishna, S. Singh, P. Singh, S. B. Rai and R. Maalej, Phys. Chem. Chem. Phys., 2014, 16, 22665–22676 RSC.
- O. A. Savchuk, P. H. Gonzalez, J. J. Carvajal, D. Jaque, J. Massons, M. Aguilo and F. Dıaz, Nanoscale, 2014, 6, 9727–9733 RSC.
- A. K. Singh, S. K. Singh, B. K. Gupta, R. Prakash and S. B. Rai, Dalton Trans., 2013, 1065–1072 RSC.
- L. L. Shi, H. J. Zhang, C. Y. Li and Q. Su, RSC Adv., 2011, 1, 298–304 RSC.
- K. Miyata, Y. Konno, T. Nakanishi, A. Kobayashi, M. Kato, K. Fushimi and Y. Hasegawa, Angew. Chem., Int. Ed., 2013, 52, 6413–6416 CrossRef CAS PubMed.
- B. Y. Lai, L. Feng, J. Wang and Q. Su, Opt. Mater., 2010, 32, 1154–1160 CrossRef CAS PubMed.
- S. F. León-Luis, U. R. Rodriguez-mendoza, E. Lalla and V. Lavin, Sens. Actuators, 2011, 158, 208–213 CrossRef PubMed.
- G. S. Maciel, L. D. S. Menezes, A. S. L. Gomes, C. B. de Araujo, Y. Messaddeq, A. Florez and M. A. Aegerter, IEEE Photonics Technol. Lett., 1995, 7, 1474–1476 CrossRef.
- B. Dong, D. P. Liu, X. J. Wang, T. Yang and S. M. Miao, Appl. Phys. Lett., 2007, 90, 181117 CrossRef PubMed.
- C. Li, B. Dong, S. F. Li and C. L. Song, Chem. Phys. Lett., 2007, 443, 426–429 CrossRef CAS PubMed.
- S. Q. Zhou, C. R. Li, Z. F. Liu, S. F. Li and C. L. Song, Opt. Mater., 2007, 30, 513–516 CrossRef CAS PubMed.
- S. K. Singh, K. Kmar and S. B. Rai, Sens. Actuators, A, 2009, 149, 16–20 CrossRef CAS PubMed.
- Y. Q. Lei, H. W. Song, L. M. Yang, L. X. Yu, Z. X. Liu, G. H. Pan, X. Bai and L. B. Fan, J. Chem. Phys., 2005, 123, 174710–174715 CrossRef PubMed.
- F. Wang, B. Debapriya, Y. S. Liu, X. Y. Chen and X. G. Liu, Analyst, 2010, 135, 1839–1854 RSC.
- Y. M. Yang, C. Mi, F. Y. Jiao, X. Y. Su, X. D. Li, L. L. Liu, J. Zhang, F. Yu, Y. Z. Liu and Y. H. Ma, J. Am. Ceram. Soc., 2014, 97, 1769–1775 CrossRef CAS PubMed.
- N. Marcin, K. Rajiv, Y. O. Tymish, E. J. Bergey and N. P. Prasad, Nano Lett., 2008, 8, 3834–3838 CrossRef PubMed.
- R. Dey and V. K. Rai, Dalton Trans., 2014, 111–118 RSC.
- X. C. Yu, F. Song, C. G. Zou, L. J. Luo, C. G. Ming, W. T. Wang, Z. Z. Cheng, L. Han, T. Q. Sun and J. G. Tian, Opt. Mater., 2009, 31, 1645–1649 CrossRef CAS PubMed.
- M. Quintanilla, E. Cantelar, F. Cussó and M. Villegas, Appl. Phys. Express, 2011, 4, 022601–022603 CrossRef.
- J. Zhang, S. W. Wang, T. J. Rong and L. D. Chen, J. Am. Ceram. Soc, 2004, 87, 1072–1075 CrossRef CAS PubMed.
- M. Pollnau, D. R. Gamelin, S. R. Luthi, H. U. Gudel and M. P. Hehlen, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 61, 3337–3346 CrossRef.
- X. Wu, J. P. Denis, Q. Ph and F. Pellé, Appl. Phys. B: Lasers Opt., 1993, 56, 269–273 CrossRef.
- M. A. Chamarro and R. Cases, J. Lumin., 1990, 46, 59–65 CrossRef CAS.
- A. Birkel, A. A. Mikhailovsky and A. K. Cheetham, Chem. Phys. Lett., 2009, 477, 325–329 CrossRef CAS PubMed.
- R. Kapoor, C. S. Friend, A. Biswas and P. N. Prasad, Opt. Lett., 2000, 25, 338–340 CrossRef CAS.
- J. Silver, M. I. Martinez-Rubio, T. G. Ireland, G. R. Fern and R. Withnall, J. Phys. Chem. B, 2001, 105, 948–953 CrossRef CAS.
- G. S. Yi, B. Q. Sun, F. Z. Yang, D. P. Chen, Y. X. Zhou and J. Cheng, Chem. Mater., 2002, 14, 2910–2914 CrossRef CAS.
- Y. M. Yang, C. A. Mi, F. Yu, X. Y. Su, C. F. Guo, G. Li, J. Zhang, L. Liu, Y. Z. Liu and X. D. Li, Ceram. Int., 2014, 40, 9875–9880 CrossRef CAS PubMed.
- O. Svelto, Principles of Lasers, Plenum, New York, 3rd edn, 1989 Search PubMed.
- S. A. Wade, S. F. Collins and G. W. Baxter, J. Appl. Phys., 2003, 94, 4743 CrossRef CAS PubMed.
- S. A. Wade, Ph. D. thesis, Victoria University, Melbourne, Australia, 1999.
- S. F. Collins, G. W. Baxter, S. A. Wade, T. Sun, K. T. V. Grattan, Z. Y. Zhang and A. W. Palmer, J. Appl. Phys., 1998, 84, 4649 CrossRef CAS PubMed.
- M. D. Shinn, W. A. Sibley, M. G. Drexhage and R. N. Brown, Phys. Rev. B: Condens. Matter Mater. Phys., 1983, 27, 6635–6648 CrossRef CAS.
- B. Dong, B. S. Cao and Y. Y. He, Adv. Mater., 2012, 24, 1987–1993 CrossRef CAS PubMed.
- S. F. León-Luis, U. R. Rodríguez-Mendoza, E. Lalla and V. Lavín, Sens. Actuators, B, 2011, 158, 208–213 CrossRef PubMed.
- B. S. Cao, Y. Y. He, Z. Q. Feng, Y. S. Li and B. Dong, Sens. Actuators, B, 2011, 159, 8–11 CrossRef CAS PubMed.
- C. K. Jorgensen and R. Reisfeld, J. Less-Common Met, 1983, 93, 107–112 CrossRef.
- X. R. Dong, X. Y Cui, Z. L. Fu, S. H. Zhou, S. Y. Zhang and Z. W. Dai, Mater. Res. Bull., 2012, 47, 212–216 CrossRef CAS PubMed.
- Z. J. Wu and S. Y. Zhang, J. Phys. Chem. A, 1999, 103, 4270–4274 CrossRef CAS.
- S. F. León-Luis, U. R. Rodríguez-Mendoza, E. Lalla and V. Lavín, Sens. Actuators, B, 2011, 158, 208–213 CrossRef PubMed.
- N. Nikifor and G. S. Maciel, Sens. Actuators, B, 2012, 164, 96–100 CrossRef PubMed.
- L. Li, C. F. Guo, S. Jiang, D. K. Agrawal and T. Li, RSC Adv., 2014, 4, 6391–6396 RSC.
- P. V. dos Santos, M. T. de Araujo and J. A. Gouveia-Neto, Appl. Phys. Lett., 1998, 73, 578–581 CrossRef CAS PubMed.
- J. H. Chung, J. H. Ryu, J. W. Eun, J. H. Lee, S. Y. Lee, T. H. Heo, B. G. Choi and B. G. Shim, J. Alloys Compd., 2012, 522, 30–34 CrossRef CAS PubMed.
- X. Wei, Z. G. Zhang and W. W. Cao, Opt. Lett., 2012, 37, 4865–4867 CrossRef PubMed.
- E. Saïdi, B. Samson, L. Aigouy, S. Volz, P. Löw, C. Bergaud and M. Mortier, Nanotechnology, 2009, 20, 115703–115711 CrossRef PubMed.
- N. Nikifor and G. S. Maciel, Sens. Actuators, B, 2012, 164, 96–100 CrossRef PubMed.
- C. R. Li, D. Dong, C. G. Ming and M. K. Lei, Sensors, 2007, 7, 2652–2659 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra05986k |
| This journal is © The Royal Society of Chemistry 2015 |

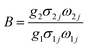
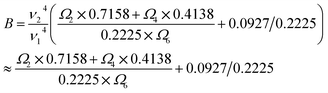
![[d with combining macron]](https://www.rsc.org/images/entities/i_char_0064_0304.gif)
![[f with combining macron]](https://www.rsc.org/images/entities/i_char_0066_0304.gif)