Microstructure, mechanical and swelling properties of microgel composite hydrogels with high microgel content and a microgel cluster crosslinker
Fang Zhao,
Xuping Qin and
Shengyu Feng*
Key Laboratory of Special Functional Aggregated Materials, Ministry of Education, College of Chemistry and Chemical Engineering, Shandong University, Jinan, 250100, P. R. China. E-mail: fsy@sdu.edu.cn; Fax: +86 531 88392430; Tel: +86 531 88392430
First published on 6th May 2015
Abstract
Acrylamide-co-2-acrylamido-2-methylpropane sulfonic acid based microgel composite (MC) hydrogels with high microgel content were prepared by post crosslinking method. Acetone was used as a demulsifier and in one group of mixed solvents, which reduced the foam during nitrogen bubbling. When the microgel content was high, the microgel particles containing hydroxymethyl groups aggregated to cluster. Acetone increased the homogeneity of cluster dispersing. The microgel composite hydrogel with high microgel content was crosslinked by a cluster, which had high mechanical strength and swelling rate. Although the tensile strength and elongation decreased as the microgel content increased, the swelling rate, the size and the number of pores increased as the microgel content increased. The exciting result was that the composite hydrogel with microgel content of 12 had a porous structure. Even though it had a high crosslinking density, the hydrogel was elastic and had excellent properties. It had a tensile strength of 44 kPa and an elongation of 45% when the water content was 90%, which is considerably higher than the conventional hydrogel. It can reach swelling equilibrium in 5 hours for 3 mm3 sizes of samples, which was faster than the conventional hydrogel owing to the partly connected porous structure. These excellent properties were due to the unique structure where the hydrogel was crosslinked by a microgel cluster. It may become a new method of preparing hydrogels with a porous structure.
1. Introduction
Hydrogels have many uses such as super absorbents, hygiene, in chemical industry and in biomedical applications. However, the scope of applications is strongly limited by their poor mechanical strength and slow swelling rate. Many cases do not require super high water absorption, but need high strength in a swollen state and a fast response rate such as separation materials, artificial muscles and other biological tissues.1,2 Although the strength of conventional hydrogels crosslinked by small molecule crosslinkers increases as the crosslinking agent increases, the hydrogel is very brittle when the crosslinking density is high.3 The strength of conventional hydrogels is considerably lower than that of the usual rubbers because of the random crosslinking structure.4,5 However, biological hydrogel tissues, such as articular cartilage, semilunar cartilage, tendons, and ligaments, have high mechanical strength.6,7 Many efforts have been focused on improving the mechanical strength of hydrogels. Hydrogels crosslinked by nanoparticles have high mechanical strength, which has gained considerable attention of many researchers.8 Nanoparticles, such as clay,3,4,9–12 hydrophobic macromolecular microsphere,13–15 hydrophilic microgels,16–20 core–shell nanospheres with hydrophobic ester groups in the core and derivable allyl groups in the shell21 and responsive activated nanogels with unsaturated double bonds,22 are reported as multifunctional crosslinkers to obtain hydrogels with high mechanical properties. It is important to note that no small molecular crosslinker is used in the hydrogel matrix and the crosslinking points are nanoparticles. The inter-crosslinking distance is proportional to the neighboring inter-particle distance.4 The evenly distributed crosslinking particles lead to the regular inter-crosslinking distance, which improves the hydrogel regularity. In addition, the load from a single broken chain will be spread over many other chains because of the multiple chains between the particles.13 The mechanical strength can be improved by the high regularity of the hydrogels and the multiple chains between the crosslinking particles.Hydrogels crosslinked by microgel particles also have a high response rate.21–25 The flexible long polymer chains between the nanogel particles and the porous structure with interconnected micropores are all beneficial to the response rate of hydrogels.22 When the amount of added microgel particles is high, the effect on the response rate is significant.24,25 Mechanical strength of composite hydrogels with double network can be significantly enhanced when the concentration of microgels reaches a certain critical value.25 In previous investigations by our groups,18,19 microgel composite (MC) hydrogels without external additives were prepared by the post crosslinking method using reactive microgels containing hydroxymethyl groups as post crosslinkers. No small molecule crosslinking agent was employed in the hydrogel matrix. The reactive microgels containing hydroxymethyl groups are previously embedded in the MC polymers. By heating MC polymers, the reactive microgels crosslink the polymer chains to form MC hydrogels. The linear polymer chains are crosslinked on the surface and inside the microgel particles.18 The microgel content is low due to a large amount of foam during the nitrogen bubbling. The microgel composite hydrogel post crosslinked by microgel particles containing hydroxymethyl groups with high microgel content has not been reported.
An accidental opportunity in our experiments was that the composite hydrogel with high microgel content was successfully prepared by adding acetone to the system. In this study, we propose preparing an MC hydrogel with high microgel content using acetone and water as the mixed solvents. Reactive microgels containing hydroxymethyl groups were used as crosslinking agents. Then, the as-prepared or the natural drying MC polymer was heated to prepare the MC hydrogel. The influence of microgel content on the microstructure and the mechanical and swelling properties of MC hydrogels were investigated.
2. Experimental section
2.1. Materials
Acrylamide (AM, 98%) was produced by Dia-Nitrix Co. (Japan). 2-Acrylamido-2-methylpropane sulfonic acid (AMPS, 99%), hydroxymethyl acrylamide (NMA, 98%) and N,N′-methylenebisacrylamide (NMBA, 99%) were purchased from Shandong Lianmeng Chemical Group Co. (China), Shandong Zibo Xinye Chemical Co. (China) and Sinopharm Chemical Reagent Co. (China), respectively. Cyclohexane (analytical reagent) was produced by Tianjin Damao Chemical Co. (China). Octylphenol ethoxylate (molecular weight 647), sorbitan monolaurate (molecular weight 182), ammonium persulfate (analytical reagent), sodium bisulfite (analytical reagent) and acetone (analytical reagent) were purchased from Shandong Shengyuanlin Chemical Co. (China). N-Methyl-2-pyrrolidone (NMP, 99%) was produced by Tianjin Kemiou Chemical Co. (China). Distilled water was used throughout the experiment.2.2. Preparation of reactive microgels containing hydroxymethyl groups
Reactive microgels containing hydroxymethyl groups were prepared by inverse emulsion polymerization. AM solutions (42.1 g AM in 55.9 ml water), AMPS (5 g), NMA (5 g), NMBA (0.005 g), sorbitan monolaurate (10.5 g) and octylphenol ethoxylate (2.5 g) were added into a 500 ml round-bottomed three-neck flask with a reflux condenser, a mechanical stirrer, and a thermometer. After being stirred for 20 min, cyclohexane (110 ml) was added into the flask. After bubbling with nitrogen for 20 min at 25 °C, the redox initiator of ammonium persulfate (0.004 g in 1 ml water) and sodium bisulfite (0.01 g in 1 ml water) was added into the system. A nitrogen atmosphere was maintained throughout the polymerization. After reacting for 3 h, the microgel emulsions were obtained.The microgel emulsions were precipitated by acetone for further use.
2.3. Preparation of MC polymer and MC hydrogel with high microgel content
MC polymer was prepared by solution polymerization. The microgel content x is the corresponding dried microgel weight in 100 g of prepolymer solutions, which is described in detail in our report.18 The water and acetone content of the prepolymer solutions is 75%. After nitrogen bubbling for 30 min, the solution was initiated by the redox initiator of ammonium persulfate (0.03 g in 0.3 ml water) and sodium bisulfite (0.01 g in 0.2 ml water) at room temperature. Then, the polymerization was carried out under airproof conditions 5 h at 40 °C and 2 h at 70 °C to obtain the MC polymer.The MC polymer was cut to an appropriate size. The natural drying polymer was obtained after 3–7 days.
The as-prepared or natural drying MC polymer was heated at 80 °C for 4 h to prepare the MC hydrogel. The hydrogels prepared by heating as-prepared MC polymers are abbreviated to MC-Ax. The hydrogels prepared by heating natural drying MC polymers are abbreviated to MC-Dx. The x is the microgel content described above.
2.4. Measurements of microgel particle size
The swollen particle size of the reactive microgels was measured by dynamic light scattering using a Malvern Zetasizer 3000 instrument (Malvern Instruments, UK) at 25 °C and at a scattering angle of 90°.2.5. Measurements of the swelling properties
The swelling experiments were performed by immersing the as-prepared hydrogels (sample size: about 3 mm × 3 mm × 3 mm) in a large excess of water at room temperature. The hydrogel was weighed every 0.5 hour on the first day and every 4 h from the second day until a constant weight was obtained, changing the water several times.The swelling ratio after t hour was calculated by using the following equation.
| Qt = (Wt − Wd)/Wd | (1) |
| Qe = (We − Wd)/Wd | (2) |
The time dependencies of the swelling ratio were represented as Qt/Qe.
2.6. Measurements of the mechanical properties of MC hydrogels
The mechanical properties of the hydrogels were measured by an electronic pull and push strength tester (MF-50, 50 N testing machine, Wenzhou Yiding Instrument Co. China). The conditions were as follows: temperature 25 °C, sample size about 5 mm (thickness) × 10 mm (width) × 50 mm (length), gauge length of 30 mm and crosshead speed 100 mm min−1. The sample used for testing had 90% water content. It was prepared as our groups reported.17 The tensile stress was calculated on the basis of the initial cross section of the specimen. The strain was defined as the change in the length with respect to the initial gauge length. Three samples were tested for each type of hydrogel and the data were averaged.2.7. Scanning electron microscopy (SEM)
The morphologies of the microgel composite hydrogels were observed using a JEOL JSM-7600F SEM after sputter-coating with platinum.3. Results and discussion
3.1. Effects of acetone
The as-prepared inverse emulsion microgels cannot redisperse in water directly because of the cyclohexane. It is necessary to remove cyclohexane by demulsification of the microgels. Many solvents such as ethanol and acetone can act as demulsifiers. The condition is that solvents can be miscible with water and cyclohexane and acrylamide hydrogels have a volume phase transition in the solvents. As shown in Fig. 1, the ternary map of cyclohexane/water/ethanol, cyclohexane/water/NMP is similar to that of cyclohexane/water/acetone. However, ethanol was not selected because ethanol and hydroxymethyl groups both contain hydroxyl groups. The hydroxyl groups of ethanol can affect the reactions of hydroxymethyl groups in the subsequent reactions. Our experiments showed that acrylamide based hydrogel absorbed water in the NMP solvents instead of the dehydrated phase transition. When NMP was used as the demulsifier, cyclohexane was difficult to remove from the system because the demulsified system was similar to the suspension state and the microgel particles were difficult to precipitate. Therefore, acetone was used as the demulsifier in our experiments.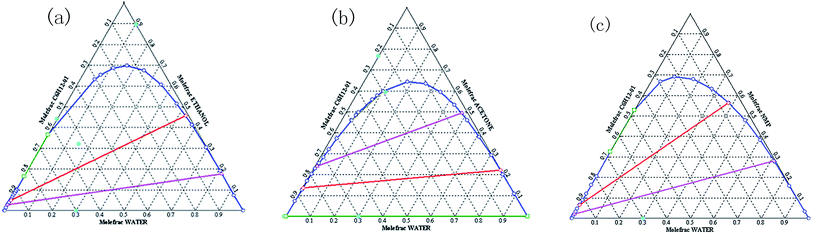 | ||
| Fig. 1 Ternary map for (a) cyclohexane/water/ethanol, (b) cyclohexane/water/acetone, and (c) cyclohexane/water/nmp (plotted by aspen plus). | ||
We found that the drying microgel particles cannot redisperse in water. The microgels contain hydroxymethyl groups, which lead to the crosslinking of the microgel particles during the drying process. The demulsified microgels were used directly without drying in our experiments.
Our experiments also showed that when the microgel content exceeded 1.0, it generated a large amount of foam in the nitrogen bubbling process, which made it difficult to do the experiment, because a lot of liquid was blown out of the beaker with the foam. However, when the acetone was added to the system, the foam reduced in the bubbling process because acetone is an organic solvent.
In addition, the demulsified microgels were difficult to disperse in pure water. As described above, the demulsified microgels were used directly in the wet state. Although the majority of cyclohexane is removed by acetone, the microgels still contain some water. As shown in Fig. 1, acetone also increases the solubility of cyclohexane in water. In other words, a small amount of cyclohexane still exists in the microgel. Therefore, it is difficult for the wet microgels to redisperse in pure water directly. Thus, mixed solvents were used.
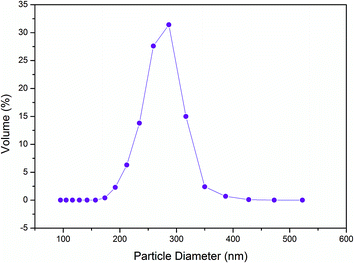
The average diameter of the microgel dispersions with microgel content of 4 is about 11.92 μm in pure water and 10.34 μm in mixed solvents with a weight of 14% acetone (Fig. 2). The particle size decreases slightly in the mixed solvents because of the volume phase transition of acrylamide hydrogels in acetone. As shown in Fig. 2, a peak corresponding to the particle size of 30–60 μm in pure water does not exist in the mixed solvents. It is shown that the microgels disperse more homogeneously in mixed solvents than in pure water. The mixed solvent is more suitable than pure water because of the high dispersing rate and more homogeneity.
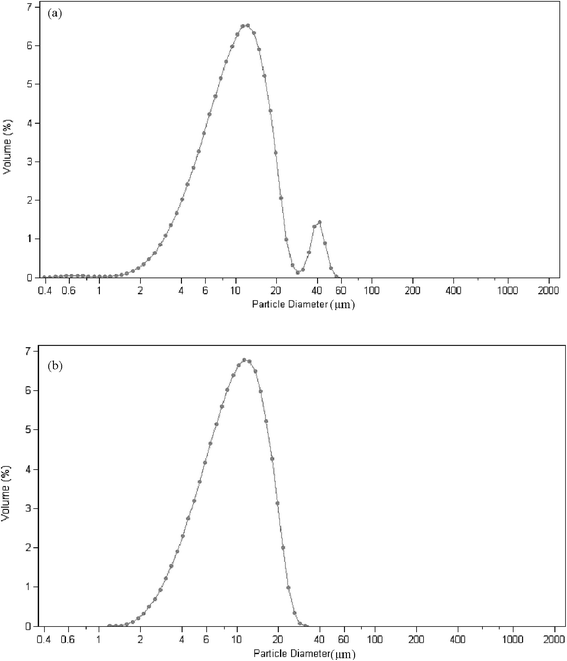 | ||
| Fig. 2 The swollen microgel particle diameter with high microgel content (a) in water (mean 11.92 μm) and (b) mix solvents with a weight of 14% acetone (mean 10.34 μm). | ||
The particle size with microgel content of 4 (Fig. 2) is considerably larger than 271 nm of the dilute dispersions with microgel content of 0.05 (Fig. 3). It is clear that microgel particles aggregate to cluster when the microgel content is high. The Woodcock equation26 is used to estimate the amorphous packing volume factor in the cluster.
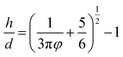 | (3) |
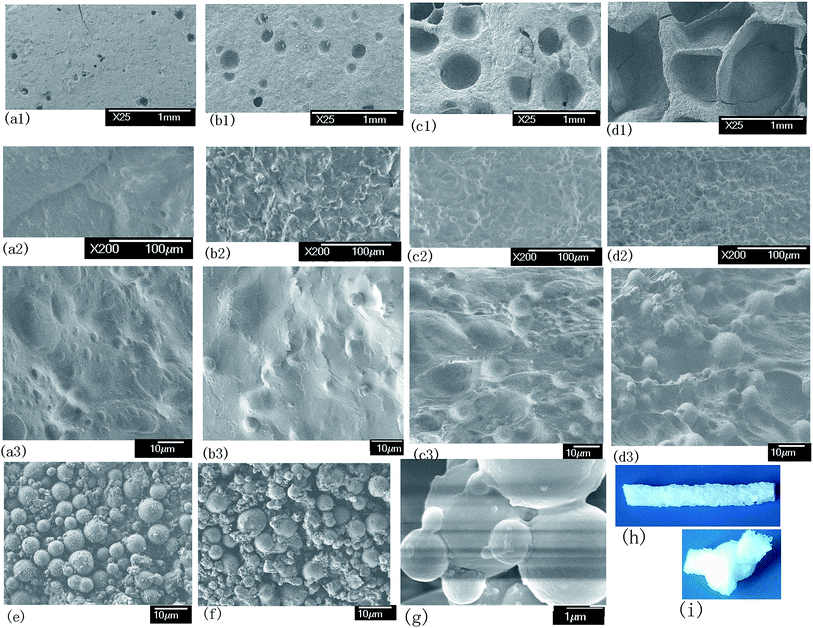
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 particles. Because the microgels contain hydroxymethyl groups, when the microgel content is high, the aggregation of microgel particle leads to the formation of the microgel cluster. The microgel particle aggregation is evident (Fig. 4g). As shown in Fig. 4, clusters are evident and distributed homogeneously in the MC hydrogels.
000 particles. Because the microgels contain hydroxymethyl groups, when the microgel content is high, the aggregation of microgel particle leads to the formation of the microgel cluster. The microgel particle aggregation is evident (Fig. 4g). As shown in Fig. 4, clusters are evident and distributed homogeneously in the MC hydrogels.
Table 1 is the hydrogels prepared by heating MC polymers with microgel content 4. The swelling ratio of MC-A hydrogels increases as the acetone content increases. It is shown that acetone can reduce the crosslinking density of MC-A hydrogels. However, the swelling ratio of MC-D hydrogels is almost not affected by the acetone, because the acetone volatilizes easily during the drying process.
3.2. Effects of microgel content
The stress–strain curves of the MC-A hydrogels with high microgel content are shown in Fig. 5 and summarized in Table 2. The samples of the MC hydrogels used for mechanical test have 90% water content.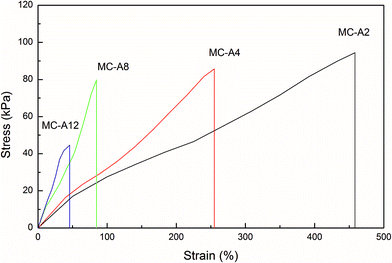 | ||
| Fig. 5 The stress–strain curves of MC-A hydrogels with different microgel content prepared by heating as-prepared MC polymers. | ||
| Hydrogela | h/db | Swelling ratio, g/g | Tensile strength, kPa | Tensile elongation, % | σ at αc, kPa | Estimated νe/100 nm3 | Modulus, kPa |
|---|---|---|---|---|---|---|---|
| a The number refers to the corresponding dried microgel weight x g of 100 g prepolymer solutions.b h/d is the first neighboring cluster/the cluster size in the as-prepared MC polymers.c The α value is in brackets. | |||||||
| MC-A2 | 0.14 | 219.2 | 94 | 458 | 15.1 (1.45) | 4000 | 34 |
| MC-A4 | 0.03 | 105 | 86 | 255 | 18.1 (1.45) | 5000 | 40 |
| MC-A8 | 0 | 31.3 | 80 | 84 | 34.5 (1.45) | 9000 | 77 |
| MC-A12 | 0 | 28.3 | 44 | 45 | 43.8 (1.45) | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
97 |
| MC-D2 | 0.14 | 17.5 | 45 | 100 | 40 (1.34) | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
118 |
| MC-D4 | 0.03 | 10.7 | 128 | 35 | 128 (1.34) | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
376 |
| MC-D8 | 0 | 8.5 | — | — | — | — | |
| MC-D12 | 0 | 14.4 | — | — | — | — | |
As the microgel content increases from 2 to 12, the tensile strength of the MC-A hydrogels decreases from 94 kPa to 44 kPa. However, the tensile elongation also reduces from 458% to 45%. This phenomenon is not consistent with the strength-elongation law of the conventional hydrogel in which the elongation and tensile strength have an inverse relation.1 It is shown that the mechanical properties of MC hydrogels with high microgel content have a different mechanism from the conventional hydrogel.
The crosslinking density is estimated from the classical equation as follows.3,4
| σ = νkT(α − α−2) | (4) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 per 100 nm3 as the microgel content increases from 2 to 12. The results are supported by the fact that the swelling ratio decreases as the microgel content increases, as shown in Table 2. The high crosslinking density was also confirmed by the fact that the modulus of hydrogels increases as the microgel content increases (Table 2). However, more crosslinking chains do not lead to higher tensile strength.
000 per 100 nm3 as the microgel content increases from 2 to 12. The results are supported by the fact that the swelling ratio decreases as the microgel content increases, as shown in Table 2. The high crosslinking density was also confirmed by the fact that the modulus of hydrogels increases as the microgel content increases (Table 2). However, more crosslinking chains do not lead to higher tensile strength.
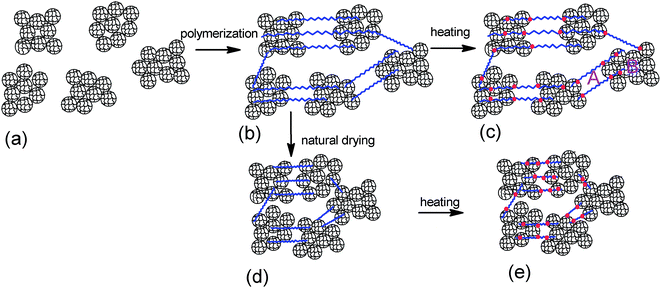
The crosslinking center of the composite hydrogel with high microgel content is not single particles but clusters formed by a lot of particles, as shown in Scheme 1. The role of the clusters is equivalent to big particles. The hydrogel contains linear parts (as marked A in Scheme 1) and cluster parts (as marked B in Scheme 1). The linear parts are similar to nanocomposite gels, which have multiple polymer chains between the clusters and regular inter-crosslinking distance caused by the homogeneously distributed clusters. These clusters are named regular parts and contain a lot of microgel particles. Microgel particles are prepared with the conventional crosslinkers NMBA. The network structure of microgel particles themselves is irregular caused by the random distribution of crosslinking points, which is similar to conventional hydrogels. Therefore, the structure of the microgel clusters themselves is irregular, which are named irregular parts. The hydrogel has regular structure parts between the clusters and irregular structure parts inside the cluster. SEM images of freeze-dried hydrogel and microgel dispersions shows that the number of clusters increases as the microgel content increases (Fig. 4). As the microgel content increases, the volume fraction of the cluster and the irregular structure parts increase, which will reduce the mechanical strength.
In addition, our experiments showed that the pores of the hydrogel increases as the microgel content increases. Fig. 4 shows the SEM morphology and a hydrogel photo. It can be evidently seen that the size and number of pores increases as the microgel content increases. The MC hydrogel with microgel content of 12 is porous. This is an exciting result. A porous structure can be obtained by adding a large amount of microgel. As the microgel content increases, the microgel can prevent the gas diffusion because of the cluster. The absorption of gas is increased by the microgels. A great quantity of gas leads to the porous structure during the hydrogel formation process. The porous structure is another factor for reducing the mechanical strength as the microgel content increases.
Fig. 6 shows the swelling rate of the MC-A hydrogels. The water absorption rate is quicker than that of the conventional hydrogels. They take up to two days to reach the equilibrium state. The swelling rate increases as the microgel content increases. The MC-A hydrogel with a microgel content of 12 has a fast swelling rate. The hydrogel can reach swelling equilibrium in 5 hours. The swelling rate of the MC-D hydrogels also increases as the microgel content increases (Fig. 7). The MC-D hydrogel with a microgel content of 12 also has a fast swelling rate. It can reach swelling equilibrium in 2 hours. It is shown that the microgel particles and the porous structure increase the swelling rate. The prevention of skin formation can accelerate the phase transition of the poly(N-isopropylacrylamide) gel.27 Similarly, the pores of the hydrogels play a role in accelerating the swelling ratio. The swelling ratio of the MC-D hydrogel with microgel content of 12 is higher than that of the MC-D hydrogel with microgel content of 8 (Table 2). This abnormal increase is also due to the porous structure.
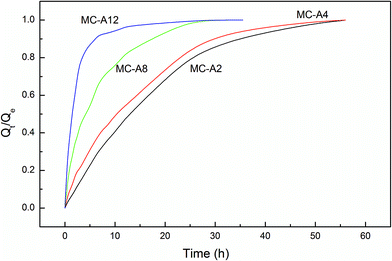 | ||
| Fig. 6 The swelling kinetics of MC-A hydrogels with different microgel content prepared by heating as-prepared MC polymers. | ||
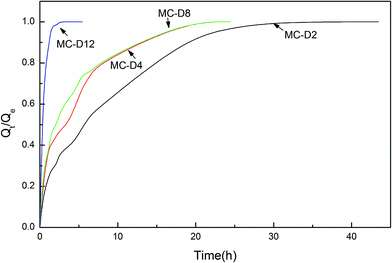 | ||
| Fig. 7 The swelling kinetics of MC-D hydrogels with different microgel content prepared by heating natural drying MC polymers. | ||
However, the swelling rate of MC-A hydrogels with microgel content of 12 is slower than that of the superporous hydrogels with open connected channels.28 As shown in Fig. 4, although the hydrogel has a porous structure, only a small amount of pores are connected by cracks and collapse. This is the reason for the porous structure having a lower swelling ratio than superporous hydrogels.
The elongation character relates to the regular parts. The NSG hydrogels are more elastic with larger elongation when longer and more flexible bridge chains are generated connecting the nanogels.22 As shown in Scheme 1, the hydrogel contains regular parts with elastic polymer chains. It leads to the elastic properties of the hydrogel. The average distance, h, of the first neighboring cluster in the as-prepared MC polymers is also estimated by the Woodcock eqn (3), as described above, where d and φ are the cluster size and the microgel cluster volume fraction, respectively. As the microgel content increases, the volume fraction increases and the distance between the cluster decreases (Table 2). Therefore, the polymer chain length between the microgels reduces, which leads to the low elongation.
The swelling ratio of conventional hydrogels crosslinked by NMBA decreases and the brittle character increases. When the conventional hydrogel crosslinked by NMBA is 5400 per 100 nm3, the hydrogel is very brittle and the tensile strength cannot be obtained because it is difficult to clamp the hydrogel.3 The calculated crosslinking density of MC-A hydrogels with microgel content of 12 is 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 per 100 nm3 (Table 2), which is considerably more than the 5400 per 100 nm3. However, the hydrogel is elastic and can knot, as shown in Fig. 4. It has a tensile strength of 44 kPa and elongation of 45% (Table 2 and Fig. 5). It is considerably higher than the conventional AM hydrogels (tensile strength of 14 kPa and elongation of 35%).15
000 per 100 nm3 (Table 2), which is considerably more than the 5400 per 100 nm3. However, the hydrogel is elastic and can knot, as shown in Fig. 4. It has a tensile strength of 44 kPa and elongation of 45% (Table 2 and Fig. 5). It is considerably higher than the conventional AM hydrogels (tensile strength of 14 kPa and elongation of 35%).15
The hydrogel also contains the irregular parts of the cluster. They can also affect the mechanical properties. As shown in Table 2, the h/d is 0 for the MC hydrogel with microgel content of 12. In other words, this hydrogel mainly contains irregular parts. However, the hydrogel has excellent mechanical properties, as indicated above. Sacrificed bonds were used to improve the hydrogel's mechanical properties in the double network hydrogel.29 The cluster contains the swollen microgel particles and the polymer chains, which is similar to the double network structure. During the strain, the microgel can provide sacrificial bonds and the polymer chains offer the restoring force to improve the elasticity of the hydrogels. Therefore, the microgel composite hydrogel has elastic properties even though it has high crosslinking density and main irregular structure parts.
As the microgel content increases from 2 to 4, the tensile strength of the MC-D hydrogels increases and elongation decreases (Table 2 and Fig. 8), and this is in agreement with the fact that the crosslinking density increases as the microgel content increases. However, as the microgel content further increases, the hydrogel is quite brittle. The tensile strength cannot be tested. MC-D hydrogels is prepared by naturally drying MC polymers. The length of the polymer chains between the clusters reduces due to the drying (Scheme 1). The first swollen network is suitable for sacrificing bonds.29 In the cluster, the microgel crosslinked polymer chains are in the drying state instead of the swollen state. Therefore, the MC-D hydrogel is brittle when the microgel content is high.
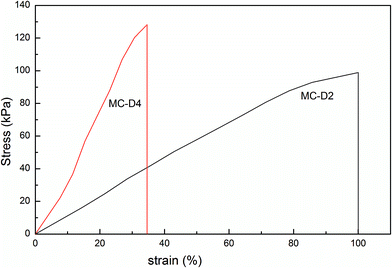 | ||
| Fig. 8 The stress–strain curves of MC-D hydrogels with different microgel content prepared by heating naturing MC polymers. | ||
4. Conclusions
MC hydrogels with high microgel content are successfully achieved by post crosslinking method. Acetone is used as the demulsifier. Mixed solvents are used as dispersing agents. Acetone reduces the foam during the nitrogen bubbling. The microgel particles aggregate to cluster when the microgel content is high. The cluster size slightly decreases and the homogeneity increases when acetone is added to the system. As acetone increases, the swelling ratio of MC-A hydrogels increases, but does not affect the swelling ratio of MC-D hydrogels.Both the tensile strength and elongation of MC-A hydrogels decrease as the microgel content increases. The MC-A hydrogels with microgel content 2 has the tensile strength of 94 kPa and the elongation of 458%. The MC-A composite hydrogel with microgel content of 12 has a porous structure. It has 44 kPa of tensile strength and 45% elongation. It can reach swelling equilibrium in 5 hours. Their excellent properties are due to the unique structure where the hydrogel is crosslinked by a microgel cluster. It contains regular structure parts and irregular structure parts. Irregular parts and the porous structure decrease the tensile strength as the microgel content increases. The swelling rate increases as the microgel content increases, also due to the porous structure. The polymer chains between the cluster and the sacrificed bonds of the microgel lead to the excellent mechanical properties. The MC-D composite hydrogel with microgel content of 12 has a high swelling rate but is brittle. It is shown that the dried microgel cannot provide sacrificial bonds.
Acknowledgements
This study was supported by Shandong Provincial Natural Science Foundation (ZR2012BM013).References
- J. Li, W. R. K. Illeperuma, Z. Suo and J. J. Vlassak, ACS Macro Lett., 2014, 3, 520–523 CrossRef CAS.
- S. Naficy, G. M. Spinks and G. G. Wallace, ACS Appl. Mater. Interface, 2014, 6, 4109–4114 CrossRef CAS PubMed.
- K. Haraguchi, R. Farnworth, A. Ohbayashi and T. Takehisa, Macromolecules, 2003, 36, 5732–5741 CrossRef CAS.
- K. Haraguchi and T. Takehisa, Adv. Mater., 2002, 14, 1120–1124 CrossRef CAS.
- Y. Tanaka, J. P. Gong and Y. Osada, Prog. Polym. Sci., 2005, 30, 1–9 CrossRef CAS PubMed.
- J. P. Gong, Y. Katsuyama, T. Kurokawa and Y. Osada, Adv. Mater., 2003, 15, 1155–1158 CrossRef CAS PubMed.
- R. E. Webber, C. C. Creton, H. R. Brown and J. P. Gong, Macromolecules, 2007, 40, 2919–2927 CrossRef CAS.
- J. Yang, J. J. Zhao, C. R. Han and J. F. Duan, Cellulose, 2014, 21, 541–551 CrossRef CAS.
- K. Haraguchi, T. Takehisa and S. Fan, Macromolecules, 2002, 35, 10162–10171 CrossRef CAS.
- K. Haraguchi and H. Li, Macromolecules, 2006, 39, 1898–1905 CrossRef CAS.
- M. F. Zhu, Y. Liu, B. Sun, W. Zhang, X. L. Liu, H. Yu, Y. Zhang, D. Kuckling and H. Adler, Macromol. Rapid Commun., 2006, 27, 1023–1028 CrossRef CAS PubMed.
- W. Zhang, Y. Liu, M. F. Zhu, Y. Zhang, X. L. Liu, Y. M. Jiang, Y. M. Chen, D. Kuckling and H. Adler, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 6640–6645 CrossRef CAS PubMed.
- T. Huang, H. G. Xu, K. X. Jiao, L. P. Zhu, H. R. Brown and H. L. Wang, Adv. Mater., 2007, 19, 1622–1626 CrossRef CAS PubMed.
- Y. T. Wu, Z. Zhou, Q. Q. Fan, L. Chen and M. F. Zhu, J. Mater. Chem., 2009, 19, 7340–7346 RSC.
- B. Kim, D. Hong and W. V. Chang, J. Appl. Polym. Sci., 2013, 130, 3574–3587 CrossRef CAS PubMed.
- X. P. Qin, F. Zhao, Y. K. Liu, H. Y. Wang and S. Y. Feng, Colloid Polym. Sci., 2009, 287, 621–625 CAS.
- X. P. Qin, F. Zhao and S. Y. Feng, eXPRESS Polym. Lett., 2011, 5, 460–469 CrossRef CAS.
- F. Zhao, X. P. Qin and S. Y. Feng, Polym. Compos., 2012, 33, 44–51 CrossRef CAS PubMed.
- F. Zhao, X. P. Qin, S. Y. Feng and Y. Gao, J. Appl. Polym. Sci., 2014, 131, 40841 Search PubMed.
- P. C. Li, K. Xu, Y. Tan, C. Lu, Y. L. Li and P. X. Wang, Polymer, 2013, 54, 5830–5838 CrossRef CAS PubMed.
- Y. Tan, K. Xu, P. X. Wang, W. B. Li, S. M. Sun and L. S. Dong, Soft Matter, 2010, 6, 1467–1471 RSC.
- L. W. Xia, R. Xie, X. J. Ju, W. Wang, Q. M. Chen and L. Y. Chu, Nat. Commun., 2013, 4, 2226 Search PubMed.
- W. S. Cai and B. R. Gupta, J. Appl. Polym. Sci., 2002, 83, 169–178 CrossRef CAS PubMed.
- J. T. Zhang, S. W. Huang, Y. N. Xue and R. X. Zhuo, Macromol. Rapid Commun., 2005, 26, 346–1350 Search PubMed.
- K. Xu, Y. Tan, Q. Chen, H. Y. An, W. B. Li, L. S. Dong and P. X. Wang, J. Colloid Interface Sci., 2010, 345, 360–368 CrossRef CAS PubMed.
- H. A. Barnes, J. F. Hutton and K. Waler, An Introduction to Rheology, Elsevier Press, Oxford, 1989 Search PubMed.
- H. Yan, H. Fujiwara, K. Sasaki and K. Tsujii, Angew. Chem., Int. Ed., 2005, 44, 1951–1954 CrossRef CAS PubMed.
- H. Omidian, J. G. Rocca and K. Park, Macromol. Biosci., 2006, 6, 703–710 CrossRef CAS PubMed.
- E. Ducrot, Y. L. Chen, M. Bulters, R. P. Sijbesma and C. Creton, Science, 2014, 344, 186–189 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |