Fabrication and characterization of carboxymethylated bael fruit gum with potential mucoadhesive applications
Atul Srivastava,
Devegowda Vishakante Gowda*,
Umme Hani,
Chetan Govindrao Shinde and
Riyaz Ali M. Osmani
Dept of Pharmaceutics, JSS College of Pharmacy, JSS University, Sri Shivarathreeshwara Nagara, Mysore-570 015, Karnataka, India. E-mail: dvgowdajssuni@gmail.com; Fax: +91 0821-2548359; Tel: +91 9482277850
First published on 23rd April 2015
Abstract
A study was conducted to enhance the mucoadhesive potential of bael fruit gum by carboxymethylation. Carboxymethylation of bael fruit gum was achieved through its reaction with monochloroacetic acid in the presence of sodium hydroxide as a catalyst under different reaction conditions. The optimal degree of substituted carboxymethyl in the carboxymethylated bael fruit gum was found to be 0.68. The resulting product was characterized by FT-IR, DSC, XRD and SEM analyses. The results revealed that the carboxymethylated derivative of bael fruit gum showed an improved mucoadhesive potential compared to unmodified gum, with a slightly increased degree of crystallinity, surface roughness and decreased viscosity. Additionally, metformin-loaded, ionotropically gelled beads of bael fruit gum and carboxymethylated bael fruit gum were formulated using calcium chloride as a cross-linking agent. An ex vivo bioadhesion study performed by a wash-off test using goat intestinal mucosa showed higher bioadhesion times for carboxymethylated bael fruit gum compared to bael fruit gum. In vitro release studies conducted using phosphate buffer (pH 6.8) showed a faster release of metformin from carboxymethylated bael fruit gum than from bael fruit gum. These results have demonstrated that carboxymethylated bael fruit gum is a promising mucoadhesive excipient.
1. Introduction
The term bioadhesion in general may be defined as the attachment or contact between two surfaces, with at least one being a biological substratum. If a mucosal layer is among the surfaces included, the term mucoadhesion is then utilized. Mucoadhesive polymers adhere to mucosal epithelial surfaces through non-specific interactions and have weak bioadhesion.1 To enhance their functional properties, natural polymers are amenable to various chemical alterations, such as graft co-polymerization, oxidation, thiolation, or carboxymethylation that result in imparting functional properties to these materials.2 Among these specified methodologies, carboxymethylation is often utilized due to its ease of processing, lower chemical cost and product versatility. Carboxymethylation of polysaccharide usually enhances the hydrophilicity and solution clarity and will result in an evident improvement in aqueous systems.3Bael fruit gum (BFG) is a non-ionic polysaccharide isolated from partially ripe fruits of Aegle marmelos, family Rutaceae. It has a backbone chain of (1 → 3)-linked, β-D-galactopyranosyl residues.4 BFG is reported to contain a high content of D-galactose (71%), arabinose (12.5%), rhamnose (6.5%) and galacturonic acid (7%). The gum exhibits an optical rotation of [α]D +84° in water.5 BFG is widely used in adhesives, gelling agents, water-proofing substances, suspending agents, thickening agents, and also as a carrier for controlled release. Due to its wide availability in nature, biocompatibility, biodegradability and non-toxicity, it represents an alluring biopolymer for a number of pharmaceutical and biomedical applications.6
Despite the fact that BFG and its derivatives have various advantages, they, like other polysaccharides, are associated with numerous drawbacks such as easier susceptibility to microbial attack, pH dependent solubility and uncontrollable rates of hydration. Carboxymethylation has emerged as a versatile modification technique not only in eliminating such drawbacks, but also to improve its swelling and solubilization behavior.7
Carboxymethylated bael fruit gum (CBFG) was characterized by Fourier transform infrared spectroscopy (FT-IR), differential scanning calorimetry (DSC), X-ray diffraction (XRD) and scanning electron microscopy (SEM) studies.
The degree of carboxymethyl substitution was determined in order to study the properties and quality of BFG, in addition to the molecular weight, purity and crystallinity.8 Diverse methods, like coulometric, conductometric and acid wash methods,9 could be implemented to aid in determining the degree of substitution. In the present work, the degree of carboxymethyl substitution was determined by a classical acid wash method.10
The mucoadhesive performance of CBFG was assessed by synthesizing mucoadhesive beads. CBFG was compared against BFG in an ex vivo bioadhesion study using freshly excised goat intestinal mucosa. Furthermore, the beads of BFG and CBFG were compared for their % entrapment, in vitro release and swelling behavior.
Metformin, an anti-diabetic drug, therapeutically utilized for the management of type 2 diabetes, has been used as a model drug because it has a dose dependent, saturable transport with absorption limited to the upper part of the intestine. Previous studies have investigated the oral delivery of metformin using other bioadhesive polymers.11
The objective of our study was to enhance the mucoadhesive properties of BFG by synthesizing a CBFG conjugate and to test it through ex vivo and in vitro studies using metformin as a model drug.
2. Experimental
2.1 Materials
Unripe bael fruit was obtained from the local market. Monochloroacetic acid and calcium chloride were purchased from Merck Specialities Pvt. Ltd., Mumbai, India. Metformin was obtained as a gift sample from Bal Pharma Ltd., Bangalore. All other chemicals used were of analytical grade and obtained commercially.2.2 Extraction of bael fruit gum (BFG)
The BFG was separated from the partially ripe bael fruits using the method reported by Jindal et al., with some modifications.12 Briefly, the gummy envelopes around each seed of the partially ripe bael fruits were collected and mixed with 2% v/v glacial acetic acid solution. The suspension was placed in a water bath for 45 min with continuous stirring and left overnight. The suspension was filtered using a nylon cloth to remove debris. To the clear filtrate, an excess of acetone was added whereupon a brownish precipitate appeared. Finally, the precipitate was dried at 50 °C under vacuum. The BFG was obtained as a light brown fine powder.2.3 Carboxymethylation of bael fruit gum (CBFG)
The method used is based on a procedure described by Dodi et al., with some modifications.13 Briefly, an aqueous dispersion of BFG (1.25%, w/v) was dispersed slowly in ice cold sodium hydroxide (45%, w/w) with vigorous stirring at 0–8 °C for 30 min. To the above solution, monochloroacetic acid (45%, w/v) was added with continuous stirring for 15 min. The reaction mixture was then heated in a thermostatic water bath at 75 °C for 1 h. The contents of the flask were shaken occasionally during the course of the reaction. The resulting mass, after cooling, was suspended in methanol (80%, v/v) and the suspension was then neutralized with glacial acetic acid. Finally, the mass was washed with methanol and dried initially at room temperature and then in a vacuum oven at 70 °C for 60 min.2.4 Characterization of CBFG
The degree of substitution (DS) of CBFG was calculated using the following equation:
 | (1) |
2.5 Preparation of BFG and CBFG beads
Ionotropically gelled beads of BFG and CBFG were prepared using metformin as a model drug and calcium chloride as a cross-linking agent. Briefly, 5% w/v of BFG or CBFG sample was dispersed in an aqueous medium containing 0.5% w/v of metformin. The obtained dispersion was extruded through a #18G needle into an aqueous solution containing 5–20% w/v of calcium chloride. The gelled beads were allowed to cross-link for 5 min followed by washing with distilled water and filtration. Afterwards, the beads were frozen for 4 h at −80 °C and then dried in a freeze dryer (Alpha 2-4 LD Plus, Martin Christ, Germany) at −90 °C at 0.0010 mbar for 24 h.2.6 Evaluation of BFG and CBFG beads
Metformin-loaded BFG and CBFG were characterized by their swelling behavior, entrapment efficiency, in vitro drug release behavior and ex vivo bioadhesion.The beads were then removed and blotted with filter paper and the changes in weight were measured. The degree of swelling was calculated using the following formula:
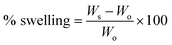 | (2) |
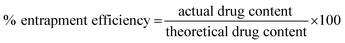 | (3) |
25 mg of beads were accurately weighed and dispersed in 100 mL of pH 6.8 phosphate buffer for 30 min. The solution was filtered through a 0.45 μm syringe filter and diluted appropriately. The drug contents of the beads were determined using a UV absorption spectrophotometer (Shimadzu 1801, USA) by measuring the absorbance at 233 nm.
3. Results and discussion
The carboxymethylation of the BFG was carried out by a Williamson’s synthesis, which is an example of an SN2 reaction.17 The main reaction proceeds in two steps.(1) In the primary reaction, sodium hydroxide deprotonates the free hydroxyl groups of the gum to provide alkoxide groups.
| BFG–OH + NaOH → BFG–ONa + H2O | (4) |
(2) The carboxymethyl groups are then formed by treating the gum alkoxides with monochloroacetic acid through an SN2 reaction.
| BFG–ONa + ClCH2COOH → BFG–OCH2COONa | (5) |
A side reaction also occurs simultaneously that results in the formation of sodium glycolates from sodium hydroxide and sodium monochloroacetate.
| NaOH + ClCH2COONa → HOCH2COONa + NaCl | (6) |
The side reaction is considerably slower than the main reaction and can be neglected for the conditions applied in this study.
Fig. 1 shows the FT-IR spectrum of the BFG and CBFG samples in the frequency region between 4000–400 cm−1. The spectrum of BFG shows a broad absorption band at 3442 cm−1 that corresponds to the –OH stretching band of a hydroxyl group, a peak at 2927 cm−1 is attributed to the C–H stretching of an alkane, and peaks at 1620 cm−1 and 1422 cm−1 are due to asymmetrical and symmetrical C–O stretching of a carboxylic acid. A band at 1046 cm−1 depicts the stretching vibration of the C–O group which is characteristic of polysaccharides.12
The spectrum of CBFG shows a broad absorption band at 3425 cm−1 attributed to –OH stretching indicating that some –OH groups were not carboxymethylated. It is a subtle difference, but the 3425 cm−1 band shows that –OH groups are present. Moreover, the BFG and CBFG curves are different in both width and height indicating that some of the –OH groups reacted. The absorption band located at 2975 cm−1 corresponds to CH groups stretching. The asymmetrical and symmetrical C–O stretching of the carboxylic acid group was assigned to 1644 and 1439 cm−1, while the C–O stretch of the carboxylic acid appears at 1059 cm−1. These bands confirm the carboxymethylation of BFG and are in agreement with the literature.2
The thermal properties of BFG and CBFG were investigated using Differential Scanning Calorimetry. Fig. 2 represents the DSC thermograms of BFG and CBFG. The DSC curve of BFG shows a broad endotherm at 105.3 °C with a heat of fusion of 342.6 J g−1. The thermal curve of CBFG shows a broad endothermic peak at 118.65 °C with a heat of fusion of 312.3 J g−1. The shift in the endothermic peak and variation in the heat flow provided more proof for the insertion of the carboxymethyl group.
Fig. 3 shows the XRD spectra of BFG and CBFG. The X-ray diffractogram of BFG is typical of amorphous materials with no sharp peaks, while the diffractogram of CBFG shows characteristic peaks at 21.6, 29.5, 37.5, 44.6, 52.3 (2θ) scale. A similar kind of observation has been reported by Kumar et al.14 for gum kondagogu, and Ahuja et al.18 for xanthan gum. The peak intensity of CBFG is slightly greater which indicates an increase in crystallinity over BFG.
Fig. 4 reveals the shape and surface morphology of the BFG and CBFG particles using scanning electron microscopy. It can be observed from the photomicrographs that the BFG and CBFG particles are polyhedral in shape (Fig. 4A and B). The surface morphology of native BFG (Fig. 4C) was observed to have an irregular but smooth surface. As can be observed, modifying the conditions brought noticeable changes to the structure of CBFG and some of the particles get attached by adhering themselves. The morphology of CBFG particles (Fig. 4D) revealed a rougher, more porous surface compared to BFG. The CBFG particles have this rough porous surface due to the cross-linking of BFG. There are various small alveolate holes on the surface of CBFG which look like surface corrosion. The alkaline treatment employed during the carboxymethylation process is responsible for these structural changes. This result also suggests that the crystallinity of BFG was altered due to the loss of crystalline structure arising from the strong alkaline conditions.
 | ||
| Fig. 4 SEM photomicrographs showing the shape of (A) BFG, (B) CBFG and the surface of (C) BFG, (D) CBFG. | ||
The degree of carboxymethyl substitution in BFG was found to be 0.68 of a carboxymethyl group per g as determined by the classical acid wash method. The main factors affecting the DS value in the CBFG synthesis reaction were investigated. These include the molar ratio of sodium hydroxide to monochloroacetic acid (mNaOH/mMCA), the volume of 70% methanol (v/v), the reaction temperature and the time of the first and second reaction steps. Taking into consideration the fact that the molar ratio of sodium hydroxide to monochloroacetic acid would distinctly affect the reaction rate and the DS of CBFG, the molar ratios of sodium hydroxide to monochloroacetic acid were varied and its influence on the DS is quoted in Table 1.
| Formulation code | Molar ratio (NaOH/MCA) | 70% methanol (v/v) | Temperature (T1, °C) | Time (t1, min) | Temperature (T2, °C) | Time (t2, min) | Degree of substitution |
|---|---|---|---|---|---|---|---|
| CBFG-A | 0.4 | 20 | 35 | 45 | 60 | 15 | 0.271 |
| CBFG-B | 0.5 | 20 | 35 | 45 | 60 | 15 | 0.486 |
| CBFG-C | 0.6 | 20 | 35 | 45 | 60 | 15 | 0.68 |
| CBFG-D | 0.7 | 20 | 35 | 45 | 60 | 15 | 0.623 |
| CBFG-E | 0.6 | 18 | 35 | 45 | 60 | 15 | 0.516 |
| CBFG-F | 0.6 | 19 | 35 | 45 | 60 | 15 | 0.561 |
| CBFG-G | 0.6 | 21 | 35 | 45 | 60 | 15 | 0.479 |
| CBFG-H | 0.6 | 22 | 35 | 45 | 60 | 15 | 0.443 |
| CBFG-I | 0.6 | 20 | 30 | 45 | 60 | 15 | 0.485 |
| CBFG-J | 0.6 | 20 | 40 | 45 | 60 | 15 | 0.461 |
| CBFG-K | 0.6 | 20 | 45 | 45 | 60 | 15 | 0.425 |
| CBFG-L | 0.6 | 20 | 35 | 40 | 60 | 15 | 0.543 |
| CBFG-M | 0.6 | 20 | 35 | 50 | 60 | 15 | 0.518 |
| CBFG-N | 0.6 | 20 | 35 | 60 | 60 | 15 | 0.478 |
| CBFG-O | 0.6 | 20 | 35 | 45 | 40 | 15 | 0.386 |
| CBFG-P | 0.6 | 20 | 35 | 45 | 50 | 15 | 0.528 |
| CBFG-Q | 0.6 | 20 | 35 | 45 | 70 | 15 | 0.512 |
| CBFG-R | 0.6 | 20 | 35 | 45 | 60 | 5 | 0.387 |
| CBFG-S | 0.6 | 20 | 35 | 45 | 60 | 10 | 0.476 |
| CBFG-T | 0.6 | 20 | 35 | 45 | 60 | 20 | 0.412 |
With the increment of mNaOH/mMCA, the DS increases to the maximal value of 0.68; however, when the ratio is more than 0.6, the DS decreases. This could be for the reason that increasing the mNaOH/mMCA ratio could lead to enhanced NaOH consumption, whereas under the present reaction conditions the NaOH amount was unchanged.19
During the carboxymethylation process, as well as serving as a swelling agent, NaOH provides an alkaline environment to facilitate the diffusion and penetration of the etherifying agent to the granular structure of BFG. The more monochloroacetic acid there is, the less sodium hydroxide can react with BFG, so that a higher mNaOH/mMCA ratio leads to a lower DS.20 The effect of the solvent medium on the extent of reaction is related to its miscibility, its ability to solubilize the etherifying agents and to swell the biopolymer, and to its ability to create an environment that favors carboxymethylation rather than glycolate formation (eqn (6)). In this work, for the BFG carboxymethylation process, 70% methanol is used as a reaction medium.
Table 1 shows that the DS increased as the volume of 70% methanol increased from 18 to 20 mL; after that a sharp decline was noted. The solvent content significantly affects the diffusion and absorption of the etherifying reagent. Additionally, the swelling of BFG is also dependent on the solvent content and this in turn increases the surface area for the reaction. The preliminary amplification in the DS accounts for these aspects. On the other hand, a higher solvent content leads to agglomeration which reduces contact between the etherifying agent and BFG molecules, consequently leading to a smaller DS.
Furthermore, the BFG carboxymethylation reaction was carried out at various temperatures to assess its effect. The outcomes show that with a rise in reaction temperature, the DS increased noticeably followed by a decline, independent of reaction step (Table 1). An increase in temperature enhanced the ionic mobility of the solutes in solution and also facilitated both the swelling of the BFG molecules and the diffusion of the reactants.21 The proportion of molecules possessing a higher energy than the activation energy rises with the rise in temperature, subsequently ensuring an augmented reaction rate and DS.22 However, it was observed that the DS reduced at temperatures higher than 35 °C; which could be attributed to volatilization of the reaction medium.
The effect of the reaction time of the first and second steps on the DS was scrutinized. The DS increases with the increase in reaction time and reaches a maximum; a significant decrease is observed on prolonging the time (Table 1). The enhancement in the DS by prolonging the duration of the reaction is a direct consequence of the favorable effect of time on the swelling of BFG and the diffusion and adsorption of the reactants, with the ultimate effect of a better contact between the etherifying agents and BFG. Similar results have been found earlier by many researchers.23 However, a longer time resulted in no further increase in the DS. Several researchers hypothesised that etherifying agents have a maximum availability independent of extended reaction time.
The viscosity profile is generally considered as one of the important parameters to evaluate the feasibility of any gum or its derivatives in industry. Fig. 5 shows the viscosity–shear rate profile of the BFG and CBFG solutions. It can be observed that the apparent viscosity decreases with an increase in the rate of shear. The viscosity of CBFG was less than that of BFG. Similar results were earlier reported for the carboxymethylation of cellulose24 and xanthan gum.18
 | ||
| Fig. 5 Rheological behavior of aqueous solutions of BFG and CBFG (each point represents mean ± SD of three replicates). | ||
The low viscosity of the CBFG solution may be attributed to a non-specific degradation of the reducing sugar unit, by β-elimination and/or peeling reaction during the carboxymethylation process. The viscosity of the BFG and CBFG solutions was observed to decrease slowly with an increase in the rate of shear from 1–7 rpm. Upon further increasing the rate of shear from 7 to 30 rpm, the viscosity decreased rapidly. No further decrease in viscosity was observed for the BFG and CBFG solutions when the rate of shear was increased from 30 to 100 rpm.
In the present study, CBFG was formulated as beads to exploit its mucoadhesive application using metformin as a model drug. As the cross-linker concentration was increased from 5–20%, the drug entrapment was found to be higher as a result of a higher degree of cross-linking between CBFG and calcium chloride, resulting in a more viscous gelation and a higher degree of drug entrapment. Taking this into consideration, 20% calcium chloride was used to synthesize the metformin loaded beads. The entrapment efficiency of metformin in BFG and CBFG was found to be 22.56% and 31.23%, respectively.
The SEM photomicrographs revealed that the metformin-loaded BFG beads were nearly spherical in shape with a smooth surface (Fig. 6A), however the shape of the metformin-loaded CBFG beads was distorted with a non-uniform and rough porous surface (Fig. 6B).25
Table 2 summarizes the results of the ex vivo bioadhesion studies performed using goat intestinal mucosa by a wash-off method. It is evident from the results that the beads synthesized using BFG showed 74% bioadhesion while the CBFG beads showed 87% bioadhesion after 24 h of study. Thus, the carboxymethylation of BFG results in improved mucoadhesive characteristics which can be further utilized to develop mucoadhesive dosage forms.
| Time (h) | % mucoadhesion of BFG | % mucoadhesion of CBFG |
|---|---|---|
| 0 | 100 ± 0.24 | 100 ± 0.35 |
| 2 | 96 ± 0.41 | 98 ± 0.33 |
| 4 | 93 ± 0.19 | 97 ± 0.27 |
| 8 | 88 ± 0.32 | 95 ± 0.18 |
| 16 | 81 ± 0.21 | 91 ± 0.31 |
| 24 | 74 ± 0.26 | 87 ± 0.28 |
The swelling study revealed that the metformin-loaded beads of BFG hydrated quickly having a swelling of 62.4% in the first hour compared to the beads synthesized using CBFG which showed a swelling of 25.8%. The reduced swelling behavior of CBFG may be due to its ability to interfere with the free access of water to the hydroxyl group of BFG. Furthermore, the beads of BFG eroded at a faster rate than the CBFG beads. The faster swelling and erosion of the BFG beads could be one of the reasons for their short bioadhesion time.
Fig. 7 represents the in vitro release profile of metformin from the BFG and CBFG beads. It was observed during our studies that CBFG showed a biphasic release pattern i.e. burst release and slow-sustained release. This indicated a combined effect of diffusion and erosion mechanisms for controlled drug release. An initial burst release of drug from the CBFG beads was observed with 30% of the drug released within the first 30 min for immediate effect followed by slow release of the drug over a prolonged period of time for a longer duration of action. The initial burst release of drug from the CBFG beads may be attributed to the decreased viscosity of CBFG. Due to the decrease in viscosity, a greater amount of drug adsorbs onto the surface of the CBFG beads during the gelation of the beads. The slow-sustained release phase could be attributed to the degradation by erosion or by hydrolysis of the CBFG beads. It can be observed from the results that the release rate is almost similar with both the formulations releasing 50% of the drug in 24 h. Thus carboxymethylation of BFG provides a means of enhancing the bioadhesion time without affecting the release rate.
4. Conclusions
In this study BFG was modified successfully to obtain its carboxymethylated derivative, which was confirmed by FT-IR spectroscopy. With a view to optimize the synthesis conditions for a higher degree of substitution, the factors that influence the synthesis, such as the ratio of monochloroacetic acid to sodium hydroxide, the volume of methanol, and the influence of the reaction time and temperature, have been investigated in this study. DSC and XRD analyses confirmed the crystalline nature of CBFG. Scanning electron microscopy revealed the rough porous surface of CBFG. The carboxymethylated derivative of BFG was found to be less viscous than BFG. Mucoadhesive applications of CBFG were explored by synthesizing ionotropically gelled beads of metformin, but further optimization of the formulation and processing variables is required. It can be concluded that CBFG is a promising bioadhesive and biocompatible polymer, which should be explored for further applications in pharmaceutical technology. The results obtained will help to design experiments to evaluate the improved bioadhesive nature of CBFG through appropriate in vivo models.Declaration of interest
The authors report no conflicts of interest.Acknowledgements
The authors express their gratitude for the financial support provided by the Dept of Science and Technology, Government of India (New Delhi, India) under grant SR/SO/HS-0107/2010 to Atul Srivastava. The authors are grateful for the excellent technical assistance of Dr Dattatri K. Nagesha, Associate Professor, Dept of Pharmaceutics, JSS College of Pharmacy, JSS University.References
- G. P. Andrews, T. P. Laverty and D. S. Jones, Eur. J. Pharm. Biopharm., 2009, 71, 505–518 CrossRef CAS PubMed.
- M. Ahuja, S. Singh and A. Kumar, Int. J. Biol. Macromol., 2013, 53, 114–121 CrossRef CAS PubMed.
- K. S. Parvathy, N. S. Susheelamma, R. N. Tharanathan and A. K. Gaonkar, Carbohydr. Polym., 2005, 62, 137–141 CrossRef CAS PubMed.
- R. K. Basak, P. K. Mandal and A. K. Mukherjee, Carbohydr. Res., 1981, 97, 315–321 CrossRef CAS.
- P. K. Mandal and A. K. Mukherjee, Carbohydr. Res., 1980, 84, 147–159 CrossRef CAS.
- N. Mahammed, D. V. Gowda, R. D. Deshpande and S. Thirumaleshwar, Arch. Pharmacal Res., 2015, 38, 42–51 CrossRef CAS PubMed.
- L. Yang, T. Zhao, H. Wei, M. Zhang, Y. Zou, G. Mao and X. Wu, Int. J. Biol. Macromol., 2011, 49, 1124–1130 CrossRef CAS PubMed.
- K. Kurita, Prog. Polym. Sci., 2001, 26, 1921–1971 CrossRef CAS.
- R. W. Eyler, E. D. Klug and F. Diephuis, Anal. Chem., 1947, 19, 24–27 CrossRef CAS.
- O. A. El Seoud, H. Nawaz and E. P. Areas, Molecules, 2013, 18, 1270–1313 CrossRef CAS PubMed.
- P. P. Ige and S. G. Gattani, Arch. Pharmacal Res., 2012, 35, 487–498 CrossRef CAS PubMed.
- M. Jindal, V. Kumar, V. Rana and A. K. Tiwary, Food Hydrocolloids, 2013, 30, 192–199 CrossRef CAS PubMed.
- G. Dodi, D. Hritcu and M. I. Popa, Cellul. Chem. Technol., 2011, 45, 171–176 CAS.
- A. Kumar and M. Ahuja, Carbohydr. Polym., 2012, 90, 637–643 CrossRef CAS PubMed.
- R. Sharma and M. Ahuja, Carbohydr. Polym., 2011, 85, 658–663 CrossRef CAS PubMed.
- C. M. Lehr, J. A. Bouwstra, J. J. Tukker and H. E. Junginer, J. Controlled Release, 1990, 13, 51–62 CrossRef CAS.
- O. S. Lawal, M. D. Lechner, B. Hartmann and W. M. Kulicke, Starch/Staerke, 2007, 59, 224–233 CrossRef CAS PubMed.
- M. Ahuja, A. Kumar and K. Singh, Int. J. Biol. Macromol., 2012, 51, 1086–1090 CrossRef CAS PubMed.
- W. Gao, X. Lin, J. Ding, X. Huang and H. Wu, Carbohydr. Polym., 2011, 84, 1413–1418 CrossRef CAS PubMed.
- W. Yanli, G. Wenyuan and L. Xia, Carbohydr. Res., 2009, 344, 1764–1769 CrossRef PubMed.
- O. S. Lawal, M. D. Lechner and W. M. Kulicke, Polym. Degrad. Stab., 2008, 93, 1520–1528 CrossRef CAS PubMed.
- L. Wang, S. Pan, H. Hu, W. Miao and X. Xu, Carbohydr. Polym., 2010, 80, 174–179 CrossRef CAS PubMed.
- P. Goyal, V. Kumar and P. Sharma, Carbohydr. Polym., 2007, 69, 251–255 CrossRef CAS PubMed.
- D. R. Biswal and R. P. Singh, Carbohydr. Polym., 2004, 57, 379–387 CrossRef CAS PubMed.
- B. Yaacob, M. C. I. Mohd Amin, K. Hashim and B. Abu Bakar, Iran. Polym. J., 2011, 20, 195–204 CAS.
| This journal is © The Royal Society of Chemistry 2015 |





