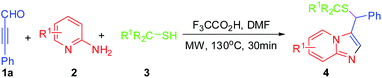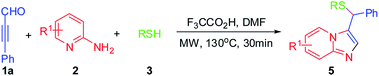Microwave-assisted C–N and C–S bond-forming reactions: an efficient three-component domino sequence for the synthesis of sulfoether-decorated imidazo[1,2-a]pyridines†
Haiying Zhan,
Hua Cao*,
Huifang Qiu,
Naiying Li,
Longbin Chen,
Jingyun Liu,
Huiyin Cai and
Jingwen Tan
School of Chemistry and Chemical Engineering, Guangdong Pharmaceutical University, Zhongshan 528458, P. R. China. E-mail: caohua@gdpu.edu.cn; Fax: +86 760 88207939
First published on 30th March 2015
Abstract
An efficient and simple microwave-assisted three-component reaction for the formation of imidazo[1,2-a]pyridine derivatives in the presence of F3CCO2H has been described. The three-component reaction of 3-phenylpropiolaldehyde, pyridin-2-amines and thiols gives the desired products in good yields. It represents an efficient approach for the formation of C–N and C–S bonds under microwave irradiation.
The formation of carbon-heteroatom bonds continues to be an active and challenging field of chemical research.1 Transition metal-catalyzed reactions have emerged as a powerful methodology for the formation of carbon-heteroatom bonds over the past decades.2 Many significant and favorable processes have been developed in this field; particularly Pd,3 Ag,4 Cu,5 Au,6 Rh (ref. 7) and Ru (ref. 8) catalysts are favorable for the formation of carbon-heteroatom bonds. However, those reactions suffer from having to use expensive catalysts and ligands. The cost, toxicity and environmental impact of these catalysts have hindered their further application on an industrial scale. These problems are of particular environmental and economic concern in large-scale syntheses. Therefore, the discovery of efficient processes that do not require a metal catalyst will be of great importance because such procedures provide an efficient route to avoid and solve these problems. It represents one of the most fundamental and economic strategies, and has attracted critical attention of organic chemists in recent years.9 In spite of the utility of transformation in the preparation of complex molecules, environmental sustainability and economy remains challenging.
Microwave-assisted chemistry10 has matured into a promising strategy for the formation of useful moleculars. It has shown tremendous advantages including simple easy work up procedure, decreased reaction time, saving energy and cost as well as providing clean products in good to excellent yields in comparison to conventional methods. Multicomponent reactions (MCRs) are very interested transformation because of their significant advantages.11 The strategy of MCRs has been developed to enable the rapid preparation of diverse structures with an optimal number of new bonds and functionalities from readily accessible starting materials in a single operation under mild conditions.
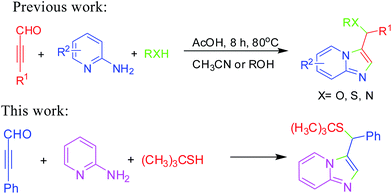
On the other hand, imidazo[1,2-a]pyridines are recognized as an important class of heterocycles which exhibited remarkable biological activities as privileged scaffolds in drug discovery and development.12 The core structure of imidazo[1,2-a]pyridines has been found in many drugs such as zolpidem, alpidem, zolimidine, olprinone, saripidem, necopidem.13 Therefore, organic chemists have been making extensive efforts to prepare imidazo[1,2-a]pyridine derivatives14 by developing novel and convenient organic transformations. Although many elegant processes have been reported to form those compounds,15 the development of new microwave-assisted domino reactions for synthesis of sulfoether-decorated imidazo[1,2-a]pyridines is still highly favorable the use of thiols and alkynes as starting materials. Our recent efforts were including the construction of imidazo[1,2-a]pyridines16 by direct C–H functionalization or multicomponent reaction. In this context, we described a novel microwave-assisted domino reactions for the formation of C–N and C–S bonds to prepare sulfoether-decorated imidazo[1,2-a]pyridine derivatives via three-component reactions of ynals, pyridin-2-amines and thiols.
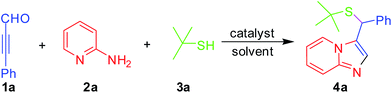
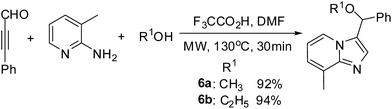
Very recently, we have developed efficient three-component reaction of ynals, pyridin-2-amines and alcohol to construct functionalized imidazo[1,2-a]pyridine (Scheme 1).16e In our further exploration of the scope of this novel domino reaction, we employed 3-phenylpropiolaldehyde, pyridin-2-amine and 2-methyl propane-2-thiol as the substrates and surprisingly found that the desired product was obtained with lower yield in the presence of AcOH in CH3CN at 80 °C for 8 h.
Initially, 3-phenylpropiolaldehyde 1a, pyridin-2-amine 2a and 2-methylpropane-2-thiol 3a were chosen as model substrates to screen the catalysts and determine suitable reaction conditions for the MCRs. The results are summarized in Table 1. The desired product 4a was formed in 10% yield in the presence of AcOH in DMF at 80 °C for 24 h(Table 1, entry 1). Interestingly, the expected product 3-(tert-butylthio(phenyl)methyl) imidazo[1,2-a]pyridine 4a was obtained in 39% yield (Table 1, entry 2) under microwave irradiation condition at 120 °C in the presence of AcOH for 30 min. Encouraged by the result that microwave irradiation could promote the reaction, other catalyst, such as PhCO2H, TsOH, F3CCO2H, HCl, and H2SO4, were next to examined (entries 3–7, Table 1). To our delight, the product 4a was formed in 83% yield in the presence of F3CCO2H under microwave heating condition. The results indicated that PhCO2H, TsOH, HCl or H2SO4 could catalyze the transformation for yielding the product in moderate yields. It was interestingly found that the three-component reaction was highly sensitive to temperature variations (Table 1, entries 8–10). When the reaction carried out decreasing the reaction temperature from 120 °C to 100 °C, the corresponding product 4a was obtained in 69% yield. Furthermore, the effects of solvents were surveyed (Table 1, entries 11–14). The results clearly indicated that best result presented when DMF was used as solvent. An increase of reaction time was studied and the same efficiency was obtained after 50 min of microwaves activation, while the decrease of reaction time lead to lower yield (Table 1, entries 15–16).
| Entry | Catalyst | Thermal/MW | Solvent | t | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1a (0.5 mmol), 2a (0.6 mmol), 3a (1.2 mmol), catalyst (2.0 mol%); solvent (3.0 mL), under microwave 500 W.b GC-yield. | |||||
| 1 | AcOH | 80 °C | CH3CN | 24h | 10 |
| 2 | AcOH | 120 °C(MW) | DMF | 30 min | 39 |
| 3 | PhCO2H | 120 °C(MW) | DMF | 30 min | 22 |
| 4 | TsOH | 120 °C(MW) | DMF | 30 min | 46 |
| 5 | F3CCO2H | 120 °C(MW) | DMF | 30 min | 83 |
| 6 | HCl | 120 °C(MW) | DMF | 30 min | 45 |
| 7 | H2SO4 | 120 °C(MW) | DMF | 30 min | 17 |
| 8 | F3CCO2H | 100 °C(MW) | DMF | 30 min | 69 |
| 9 | F3CCO2H | 130 °C(MW) | DMF | 30 min | 84 |
| 10 | F3CCO2H | 150 °C(MW) | DMF | 30 min | 76 |
| 11 | F3CCO2H | 130 °C(MW) | DMSO | 30 min | 80 |
| 12 | F3CCO2H | 130 °C(MW) | Toluene | 30 min | 34 |
| 13 | F3CCO2H | 130 °C(MW) | DMA | 30 min | 79 |
| 14 | F3CCO2H | 130 °C(MW) | Dioxane | 30 min | 36 |
| 15 | F3CCO2H | 130 °C(MW) | DMF | 20 min | 72 |
| 16 | F3CCO2H | 130 °C(MW) | DMF | 50 min | 82 |
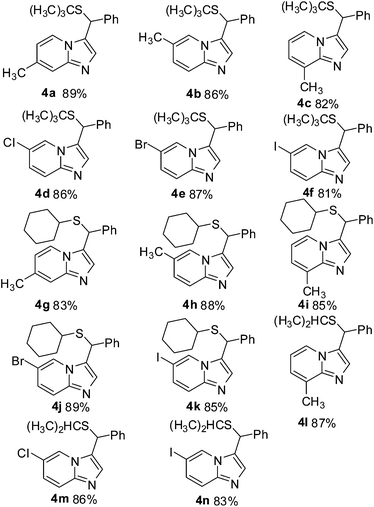
Under the optimized reaction conditions, we evaluated the scope of this novel transformation synthesis of sulfoether-decorated imidazo[1,2-a]pyridines. The results are summarized in Table 2. First, 1a and 3a fixed as substrates to test various substituted pyridin-2-amines. As shown in Table 2, a variety of substituted pyridin-2-amine derivatives were effective substrates for this transformation and the corresponding products (4a–f) were obtained in good yields in all the cases. To our delight, the sensitive functionalized groups on the pyridine ring, such as Cl, Br and I, were also tolerated to afford the corresponding in good yields. Subsequently, cyclohexanethiol (3b) and propane-2-thiol (3c) were also employed for this transformation. The results also indicated that all of the reactions proceeded smoothly under the optimized conditions and provided the thioether-decorated imidazo[1,2-a]pyridine derivatives (4g–n) in good yields.
| a Isolated yields. |
|---|
 |
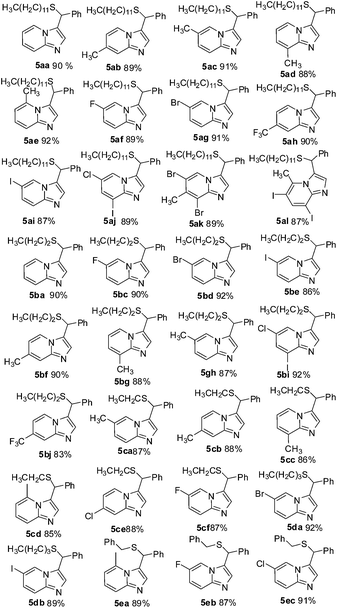
Encouraged by the successful syntheses of substituted imidazo[1,2-a]pyridines, our attention turned to the possibility of synthesizing functionalized imidazo[1,2-a]pyridine derivatives by using primary thiols in this reaction under the optimized conditions. And the results are shown in Table 3. Dodecane-1-thiol (3d) was firstly examined. Various substituted pyridin-2-amines reacted well with 1a, 3d and led to the desired product 5aa–al in good to high yields. Furthermore, multisubstituted pyridin-2-amine, such as 5-chloro-3-iodopyridin-2-amine, 3,5-dibromo-4-methylpyridin-2-amine or 3,5-diiodo-6-methylpyridin-2-amine reacted with 1a and 3d smoothly under the optimized conditions. Other commercially available aliphatic primary thiols, such as, propane-1-thiol(3e), ethanethiol (3f), butane-1-thiol (3g), and phenylmethanethiol (3f), were also tested. We were pleased to find that substituted thiols as substrates were also tolerated in the reaction and provided the desired imidazo[1,2-a]pyridines 5ba–ec in good to excellent yields. To our delight, substituted thiols reacted with 1a and multisubstituted or electron-poor groups substituted (CF3) pyridin-2-amines smoothly and afforded the desired product in good yields. It is worth pointing out that multi-halogen-substituted pyridin-2-amines could also be achieved upon carrying out the experiment under the optimized conditions. The results clearly indicated that this strategy can be extended to a variety of substituted thiols to form functionalized imidazo[1,2-a]pyridine derivatives. Notably, the sole products were detected in the reaction, which was indicated that this one pot transformation was regioselective and chemoselective.
| a Isolated yields. |
|---|
 |
To our delight, other nucleophiles, such as CH3OH and C2H5OH, were also performed very well and offered the corresponding products in 95% and 86% yields respectively (Scheme 2).
In summary, we have reported an efficient microwave-assisted three-component reactions synthesis of sulfoether-decorated imidazo[1,2-a]pyridines. This procedure offers easy access to highly functionalized imidazo[1,2-a]pyridines which are useful structural motif in pharmaceuticals and natural compounds. This transformation provides a convenient strategy for the formation of C–N and C–S bonds to prepare sulfoether-decorated imidazo[1,2-a]pyridines. This methodology has several advantages: (1) it does not require expensive substrates or catalysts; (2) it decrease reaction time, saves energy and cost as well as provides clean products in good to excellent yields; (3) it has provided a wide range of substrates. Further studies and applications of metal-free multicomponent reactions are ongoing in our laboratory.
Acknowledgements
This research was financially Supported by National Natural Science Foundation of China (21302023) and the Project of Department of Education of Guangdong Province (2013KJCX0111).Notes and references
- (a) D. G. Yu, B. J. Li and Z. J. Shi, Acc. Chem. Res., 2010, 43, 1486–1495 CrossRef CAS PubMed; (b) M. E. Doster, J. A. Hatnean, T. Jeftic, S. Modi and S. A. Johnson, J. Am. Chem. Soc., 2010, 132, 11923–11925 CrossRef CAS PubMed; (c) N. J. Turner, Chem. Rev., 2011, 111, 4073–4087 CrossRef CAS PubMed; (d) J. Escudié, H. Ranaivonjatovo and L. Rigon, Chem. Rev., 2000, 100, 3639–3696 CrossRef PubMed; (e) A. A. Kantak, S. Potavathri, R. A. Barham, K. M. Romano and B. DeBoef, J. Am. Chem. Soc., 2011, 133, 19960–19965 CrossRef CAS PubMed; (f) H. J. Kim, J. Kim, S. H. Cho and S. Chang, J. Am. Chem. Soc., 2011, 133, 16382–16385 CrossRef CAS PubMed; (g) S. H. Cho, J. Yoon and S. Chang, J. Am. Chem. Soc., 2011, 133, 5996–6005 CrossRef CAS PubMed; (h) C. J. Li, Acc. Chem. Res., 2010, 43, 581–590 CrossRef CAS PubMed; (i) W. J. Yoo and C. J. Li, J. Am. Chem. Soc., 2006, 128, 13064–13065 CrossRef CAS PubMed.
- (a) S. F. Zhu and Q. L. Zhou, Acc. Chem. Res., 2012, 45, 1365–1377 CrossRef CAS PubMed; (b) B. D. Sherry and A. Fürstner, Acc. Chem. Res., 2008, 41, 1500–1511 CrossRef CAS PubMed; (c) E. M. Beccalli, G. Broggini, M. Martinelli and S. Sottocornola, Chem. Rev., 2007, 107, 5318–5365 CrossRef CAS PubMed; (d) S. A. Girard, X. Hu, T. Knauber, F. Zhou, M. O. Simon, G. J. Deng and C. J. Li, Org. Lett., 2012, 14, 5606–5609 CrossRef CAS PubMed; (e) N. Uhlig and C. J. Li, Org. Lett., 2012, 14, 3000–3003 CrossRef CAS PubMed.
- (a) W. Q. Wu and H. F. Jiang, Acc. Chem. Res., 2012, 45, 1736–1748 CrossRef CAS PubMed; (b) S. E. Denmark and C. S. Regens, Acc. Chem. Res., 2008, 41, 1486–1499 CrossRef CAS PubMed; (c) S. I. Kuwabe, K. E. Torraca and S. L. Buchwald, J. Am. Chem. Soc., 2001, 123, 12202–12206 CrossRef CAS PubMed; (d) M. Palucki, J. P. Wolfe and S. L. Buchwald, J. Am. Chem. Soc., 1996, 118, 10333–10334 CrossRef CAS; (e) J. J. Yin, M. M. Zhao, M. A. Huffman and J. M. McNamara, Org. Lett., 2002, 4, 3481–3484 CrossRef CAS PubMed.
- (a) H. Mandai, K. Mandai, M. L. Snapper and A. H. Hoveyda, J. Am. Chem. Soc., 2008, 130, 17961–17969 CrossRef CAS PubMed; (b) R. Umeda and A. Studer, Org. Lett., 2008, 10, 993–996 CrossRef CAS PubMed.
- (a) M. Meldal and C. W. Tornøe, Chem. Rev., 2008, 108, 2952–3015 CrossRef CAS PubMed; (b) S. J. Balkrishna, B. S. Bhakuni, D. Chopra and S. Kumar, Org. Lett., 2010, 12, 5394–5397 CrossRef CAS PubMed.
- (a) A. Aponick, C. Y. Li and B. Biannic, Org. Lett., 2008, 10, 669–671 CrossRef CAS PubMed; (b) A. Aponick, C. Y. Li and J. A. Palmes, Org. Lett., 2009, 11, 121–124 CrossRef CAS PubMed; (c) J. A. Akana, K. X. Bhattacharyya, P. Müller and J. P. Sadighi, J. Am. Chem. Soc., 2007, 129, 7736–7737 CrossRef CAS PubMed; (d) A. S. Dudnik, Y. Z. Xia, Y. H. Li and V. Gevorgyan, J. Am. Chem. Soc., 2010, 132, 7645–7655 CrossRef CAS PubMed.
- (a) D. A. Colby, A. S. Tsai, R. G. Bergman and J. A. Ellman, Acc. Chem. Res., 2012, 45, 814–825 CrossRef CAS PubMed; (b) N. Guimond, C. Gouliaras and K. Fagnou, J. Am. Chem. Soc., 2012, 134, 8148–18148 CrossRef PubMed.
- (a) W. J. Yang, L. J. Xu, Z. Chen, L. L. Zhang, M. Miao and H. Ren, Org. Lett., 2013, 15, 1282–1285 CrossRef CAS PubMed; (b) B. M. Trost, H. Yang and G. Wuitschik, Org. Lett., 2005, 7, 4761–4764 CrossRef CAS PubMed; (c) M. Zhang, H. F. Jiang, H. Neumann, M. Beller and P. H. Dixneuf, Angew. Chem., Int. Ed., 2009, 121, 1709–1712 CrossRef.
- (a) Y. H. Li, L. Q. Lu, S. Das, S. Pisiewicz, K. Junge and M. Beller, J. Am. Chem. Soc., 2012, 134, 18325–18329 CrossRef CAS PubMed; (b) C. Zhu, G. Q. Li, D. H. Ess, J. R. Falck and L. Kürti, J. Am. Chem. Soc., 2012, 134, 18253–18256 CrossRef CAS PubMed; (c) J. A. Souto, P. Becker, Á. Iglesias and K. Muñiz, J. Am. Chem. Soc., 2012, 134, 15505–15511 CrossRef CAS PubMed; (d) C. M. Counceller, C. C. Eichman, B. C. Wray and J. P. Stambuli, Org. Lett., 2008, 10, 1021–1023 CrossRef CAS PubMed; (e) S. W. Kwok, J. R. Fotsing, R. J. Fraser, V. O. Rodionov and V. V. Fokin, Org. Lett., 2010, 12, 4217–4219 CrossRef CAS PubMed; (f) K. Ekoue-Kovi and C. Wolf, Org. Lett., 2007, 9, 3429–3432 CrossRef CAS PubMed; (g) C. I. Herrerías, X. Q. Yao, Z. P. Li and C. J. Li, Chem. Rev., 2007, 107, 2546–2562 CrossRef PubMed.
- (a) S. M. Ghouse, Y. S. Kumar, J. S. Jin, J. P. Kim, J. S. Bae, E. H. Chung, D. Y. Kim, E. K. Jang, F. R. N. Khan and E. D. Jeong, RSC Adv., 2014, 4, 44408–44417 RSC; (b) G. Hervéa and C. Len, RSC Adv., 2014, 4, 46926–46929 RSC; (c) S. Karamthulla, S. Pal, M. N. Khan and L. H. Choudhury, RSC Adv., 2014, 4, 37889–37899 RSC; (d) L. J. Ma and T. Inokuchi, Chem. Commun., 2010, 46, 7037–7039 RSC; (e) Akanksha and D. Maiti, Green Chem., 2012, 14, 2314–2320 RSC; (f) D. Limnios and C. G. Kokotos, RSC Adv., 2013, 3, 4496–4499 RSC; (g) A. V. Dolzhenko, S. A. Kalininac and D. V. Kalinin, RSC Adv., 2013, 3, 15850–15855 RSC; (h) D. Kundu, S. Ahammed and B. C. Ranu, Green Chem., 2012, 14, 2024–2030 RSC; (i) M. L. Clarke, Adv. Synth. Catal., 2005, 347, 303–307 CrossRef CAS; (j) F. Benfatti, G. Cardillo, L. Gentilucci, A. Tolomelli, M. Monari and F. Piccinelli, Adv. Synth. Catal., 2007, 349, 1256–1264 CrossRef CAS; (k) D. D. Vachhani, V. P. Mehta, S. G. Modha, K. V. Hecke, L. V. Meervelt and E. V. V. Eyckena, Adv. Synth. Catal., 2012, 354, 1593–1599 CrossRef CAS; (l) M. Nagaraj, M. Boominathan, S. Muthusubramaian and N. Bhuvanesh, Synlett, 2012, 23, 1353–1357 CrossRef CAS PubMed; (m) M. Adib, A. Mohamadi, E. Sheikhi, S. Ansari and H. R. Bijanzadeh, Synlett, 2010, 11, 1606–1608 CrossRef PubMed.
- (a) A. Dömling, Chem. Rev., 2006, 106, 17–89 CrossRef PubMed; (b) M. González-López and J. T. Shaw, Chem. Rev., 2009, 109, 164–189 CrossRef PubMed; (c) I. V. Kozhevnikov, Chem. Rev., 1998, 98, 171–198 CrossRef CAS PubMed; (d) V. Nair, C. Rajesh, A. U. Vinod, S. Bindu, A. R. Sreekanth, J. S. Mathen and L. Balagopal, Acc. Chem. Res., 2003, 36, 899–907 CrossRef CAS PubMed; (e) J. Yu, F. Shi and L. Z. Gong, Acc. Chem. Res., 2011, 44, 1156–1171 CrossRef CAS PubMed; (f) V. Nair, S. Bindu, V. Sreekumar and N. P. Rath, Org. Lett., 2003, 5, 665–667 CrossRef CAS PubMed.
- (a) A. A. Trabanco, G. Tresadern, G. J. Macdonald, J. A. Vega, A. I. d. Lucas, E. Matesanz, A. García, M. L. Linares, S. A. A. Diego, J. M. Alonso, D. Oehlrich, A. Ahnaou, W. Drinkenburg, C. Mackie, J. I. Andrés, H. Lavreysen and J. M. Cid, J. Med. Chem., 2012, 55, 2688–2701 CrossRef CAS PubMed; (b) G. Tresadern, J. M. Cid, G. J. Macdonald, J. A. Vega, A. I. Lucas, A. García, E. Matesanz, M. L. Linares, D. Oehlrich, H. Lavreysen, I. Biesmans and A. A. Trabanco, Bioorg. Med. Chem. Lett., 2010, 20, 175–179 CrossRef CAS PubMed; (c) R. Ducray, C. D. Jones, F. H. Jung, I. Simpson, J. Curwen and M. Pass, Bioorg. Med. Chem. Lett., 2011, 21, 4702–4704 CrossRef CAS PubMed; (d) R. Ducray, I. Simpson, F. H. Jung, W. M. Nissink, P. W. Kenny, M. Fitzek, G. E. Walker, L. T. Ward and K. Hudson, Bioorg. Med. Chem. Lett., 2011, 21, 4698–4701 CrossRef CAS PubMed; (e) C. Hamdouchi, B. Zhong, J. Mendoza, E. Collins, C. Jaramillo, J. E. Diego, D. Robertson, C. D. Spencer, B. D. Anderson, S. A. Watkins, F. Zhang and H. B. Brooks, Bioorg. Med. Chem. Lett., 2005, 15, 1943–1947 CrossRef CAS PubMed.
- (a) A. Elhakmaoui, A. Gueiffier, J. C. Milhavet, Y. Blache, J. P. Chapat, O. Chavignon, J. C. Teulade, R. Snoeck, G. Andrei and E. D. Clercq, Bioorg. Med. Chem. Lett., 1994, 4, 1937–1940 CrossRef; (b) M. Andaloussi, E. Moreau, N. Masurier, J. Lacroix, R. C. Gaudreault, J. M. Chezal, A. E. Laghdach, D. Canitrot, E. Debiton, J. C. Teulade and O. Chavignon, Eur. J. Med. Chem., 2008, 43, 2505–2517 CrossRef CAS PubMed.
- (a) A. J. Stasyuk, M. Banasiewicz, M. K. Cyrański and D. T. Gryko, J. Org. Chem., 2012, 77, 5552–5558 CrossRef CAS PubMed; (b) N. Chernyak and V. Gevorgyan, Angew. Chem., Int. Ed., 2010, 49, 2743 CrossRef CAS PubMed; (c) H. Wang, Y. Wang, D. Liang, L. Liu, J. Zhang and Q. Zhu, Angew. Chem., Int. Ed., 2011, 50, 5678–5681 CrossRef CAS PubMed; (d) S. K. Guchhait, A. L. Chandgude and G. Priyadarshani, J. Org. Chem., 2012, 77, 4438–4444 CrossRef CAS PubMed; (e) L. Ma, X. Wang, W. Yu and B. Han, Chem. Commun., 2011, 47, 11333–11335 RSC; (f) S. Santra, A. K. Bagdi, A. Majee and A. Hajra, Adv. Synth. Catal., 2013, 355, 1065–1070 CrossRef CAS; (g) A. K. Bagdi, M. Rahman, S. Santra, A. Majee and A. Hajra, Adv. Synth. Catal., 2013, 355, 1741–1747 CrossRef CAS; (h) X. Wang, K. Maeda, X. Chen, K. Takanabe, K. Domen, Y. Hou, X. Fu and M. Antonietti, J. Am. Chem. Soc., 2009, 131, 1680–1681 CrossRef CAS PubMed; (i) K. S. Masters, T. R. M. Rauws, A. K. Yasav, W. A. Herrebout, B. V. Veken and B. U. W. Mases, Chem.–Eur. J., 2011, 17, 6315–6320 CrossRef CAS PubMed; (j) C. He, J. Hao, H. Xu, Y. P. Mo, H. Y. Liu, J. J. Han and A. W. Lei, Chem. Commun., 2012, 48, 11073–11075 RSC; (k) V. Z. Parchinsky, O. Shuvalova, O. Ushakova, D. V. Kravchenko and M. Krasavin, Tetrahedron Lett., 2006, 47, 947–951 CrossRef CAS PubMed; (l) F. Chen, M. Lei and L. Hu, Tetrahedron, 2013, 69, 2954–2960 CrossRef CAS PubMed; (m) V. Tyagi, S. Khan, V. Bajpai, H. M. Gauniyal, B. Kumar and P. M. S. Chauhan, J. Org. Chem., 2012, 77, 1414–1421 CrossRef CAS PubMed.
- (a) D. M. Chandra, S. R. Nageswara and S. Adimurthy, J. Org. Chem., 2013, 78, 266–1272 Search PubMed; (b) R. L. Yan, H. Yan, C. Ma, Z. Y. Ren, X. A. Gao, G. S. Huang and Y. M. Liang, J. Org. Chem., 2012, 77, 2024–2028 CrossRef CAS PubMed; (c) S. Husinec, R. Markovic, M. Petkovic, V. Nasufovic and V. Savic, Org. Lett., 2011, 13, 2286–2289 CrossRef CAS PubMed; (d) Z. Fei, Y. P. Zhu, M. C. Liu, F. C. Jia and A. X. Wu, Tetrahedron Lett., 2013, 54, 1222–1226 CrossRef CAS PubMed; (e) L. R. Wen, C. Y. Jiang, M. Li and L. J. Wang, Tetrahedron, 2011, 67, 293–302 CrossRef CAS PubMed; (f) J. Koubachi, S. E. Kazzouli, S. Berteina-Raboin, A. Mouaddib and G. Guillaumet, J. Org. Chem., 2007, 72, 7650–7655 CrossRef CAS PubMed; (g) H. Y. Fu, L. Chen and H. Doucet, J. Org. Chem., 2012, 77, 4473–4478 CrossRef CAS PubMed; (h) J. Zeng, Y. J. Tan, M. L. Leow and X. W. Liu, Org. Lett., 2012, 14, 4386–4389 CrossRef CAS PubMed; (i) Y. Gao, M. Z. Yin, W. Q. Wu, H. W. Huang and H. F. Jiang, Adv. Synth. Catal., 2013, 355, 2263–2273 CrossRef CAS; (j) Y. Xu, X. Tang, W. Hu, W. Wu and H. Jiang, Green Chem., 2014, 16, 3720–3723 RSC; (k) E. P. A. Talbot, M. Richardson, J. M. McKenna and F. D. Toste, Adv. Synth. Catal., 2014, 356, 687–691 CrossRef CAS PubMed.
- (a) H. Cao, X. Liu, J. Liao, J. Huang, H. Qiu, Q. Chen and Y. Chen, J. Org. Chem., 2014, 79, 11209–11214 CrossRef CAS PubMed; (b) H. Cao, H. Y. Zhan, J. H. Cen, J. X. Lin, Y. G. Lin, Q. X. Zhu, M. L. Fu and H. F. Jiang, Org. Lett., 2013, 15, 1080–1083 CrossRef CAS PubMed; (c) H. Y. Zhan, X. L. Lin, Y. Y. Qiu, Z. D. Du, P. X. Li, Y. J. Li and H. Cao, Eur. J. Org. Chem., 2013, 2284–2287 CrossRef CAS; (d) H. Cao, H. Y. Zhan, Y. G. Lin, X. L. Lin, Z. D. Du and H. F. Jiang, Org. Lett., 2012, 14, 1688–1691 CrossRef CAS PubMed; (e) H. Cao, X. H. Liu, L. Zhao, J. H. Cen, J. X. Lin, Q. X. Zhu and M. L. Fu, Org. Lett., 2014, 16, 146–149 CrossRef CAS PubMed; (f) H. Cao, S. Lei, J. Liao, J. Huang, H. Qiu, Q. Chen, S. Qiu and Y. Chen, RSC Adv., 2014, 4, 50137–50140 RSC; (g) H. Cao, S. Lei, N. Li, L. Chen, J. Liu, H. Cai, S. Qiu and J. Tan, Chem. Commun., 2015, 51, 1823–1825 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental protocols and NMR spectra for products 4aa–4eg. See DOI: 10.1039/c5ra05377c |
| This journal is © The Royal Society of Chemistry 2015 |