Effects of supplemental organic carbon on long-term reduction and reoxidation of uranium†
Fubo Luan*a,
Gengxin Zhangb,
John M. Senkoc and
William D. Burgos*a
aDepartment of Civil and Environmental Engineering, The Pennsylvania State University, University Park, 212 Sackett Building, PA 16802-1408, USA. E-mail: ful6@psu.edu; wdb3@psu.edu; Fax: +1 814 863 7304; Tel: +1 814 863 0578
bInstitute of Tibetan Research, Chinese Academy of Sciences, Beijing, China
cDepartment of Geosciences and Department of Biology, The University of Akron, Akron, OH, USA
First published on 26th March 2015
Abstract
Bioreduction of mobile uranyl(VI) (UO22+) to sparingly soluble uraninite (U(IV)O2(s)) is a strategy that has been proposed for in situ remediation of uranium contaminated aquifers. That strategy faces the challenge of reoxidation of uraninite, with consequent release of soluble uranyl when the stimulation of U(VI) bioreduction is terminated. We tested the effects of supplemental organic carbon (ethanol) addition on the long-term reduction and subsequent reoxidation of uranium. In 620 days (31 pore volumes) flow-through bioreduction experiments with 1 or 10 mM ethanol, no obvious difference was observed in effluent U(VI), effluent nitrate, and effluent sulfate. However, a higher concentration of ethanol (10 mM) supported more extensive sulfate reduction to sulfide compared to lower ethanol (1 mM). Upon completion of bioreduction experiments, U(IV) in both 1 and 10 mM ethanol-fed columns was resistant to reoxidation upon addition of oxygenated water to the columns for 110 days (182 pore volumes). Columns that received a higher concentration of ethanol (10 mM) exhibited less U(IV) reoxidation in the presence of nitrate compared to 1 mM ethanol-fed column sediments, and similar results were observed in batch reoxidation experiments in which O2 was used as an oxidant. Our results demonstrate that supplemental organic carbon could protect biogenic U(IV) from remobilization upon intrusion of oxidants.
Introduction
Uranium is a common radionuclide contaminant in soils, sediments, and groundwater at uranium mining, nuclear research, and weapons manufacturing sites. In the U.S., uranium contamination has been documented in 36 states and territories.1 One strategy for the remediation of uranium-contaminated soil and groundwater is to stimulate reduction of soluble uranyl(VI) (UO22+) to sparingly soluble mineral uraninite(IV) (UO2(s)) under anoxic conditions.2–4 This strategy has been used in in situ remediation of uranium contamination.4–8 Many of these studies have focused on two Department of Energy (DOE) field research sites: the Oak Ridge, TN Field Research Center (FRC), and the Rifle, CO Uranium Mine Tailings Remediation Act (UMTRA) site. Although the hydrogeology, geochemistry, and sediment mineralogy of these two sites are quite different,4,9,10 U(VI) concentrations at both sites could be lowered below relevant standards by injection of supplemental organic carbon as an electron donor.4,6 Dissimilatory metal reducing bacteria (DMRB)11 and sulfate reducing bacteria (SRB)12 are the main bacteria responsible for uranium reduction.4,13,14Bioreduction of uranium is strongly dependent on the supplemental organic carbon supply.5,7,14 Low concentration of supplemental organic carbon (lower than 0.14 mmol per kg per day lactate or acetate) was reported to be insufficient to completely reduce and immobilize all dissolved U(VI), but relatively high concentrations of supplemental organic carbon (1.4 mmol per kg per day lactate or acetate) caused an increase in aqueous U(VI), even under reducing conditions.15 These results indicate that maintaining a proper concentration of supplemental organic carbon is an important consideration for in situ uranium remediation.
At uranium remediation sites, the injection of oxygen or nitrate caused reoxidization and remobilization of reduced uraninite when electron donor addition is terminated.5,6 Oxygen can oxidize uraninite abiotically16 while nitrate cannot.7 Nitrate oxidized uraninite through biological nitrate-dependent U(IV) oxidation pathway. A number of laboratory-based experiments have been further conducted using material from uranium-contaminated sites to better understand the stability of biogenic uraninite (details in Table 1).17–21 These studies have shown that the microbial oxidation rate of U(IV) by nitrate was faster than by oxygen even at the same electron acceptor equivalence.20 However, in the presence of sulfate, sulfate could be reduced to sulfide, which might scavenge intruding oxidants and could protect uraninite from reoxidization by oxygen and nitrate.20,22,23 Sulfide proved to be more protective of biogenic U(IV) in the presence of O2 than nitrate.20
| Reference | Filed/sediments | Organic carbon content in sediment (%) | Flow rate (mL per day)/residence time (days) | Added bacteria | Reduction experiment | Electron donor | Reoxidation experiment | |||
|---|---|---|---|---|---|---|---|---|---|---|
| U (μM) | NO3− (mM) | SO42− (mM) | U (μM) | Oxidants | ||||||
| This study | Oak Ridge FRC 2 | 0.487 ± 0.006 | 1.0/20 | — | 2 | 1.0 | 1.0 | Ethanol 1–10 mM | — | O2/NO3− |
| Ref. 17 | Old Rifle, CO | 0.17 ± 0.1 | 288/0.33 | Geobacter metallireducens | 20 | — | — | Acetate 3 mM | 20 | O2/NO3− |
| Ref. 18 | Old Rifle, CO | — | 288/0.34 | Geobacter metallireducens | 20 | — | 0.009 | Acetate 3 mM | 20 | O2 |
| Ref. 19 | Old Rifle, CO | — | 288/0.24 | Geobacter metallireducens | 20 | — | 0.009 | Acetate 3 mM | 20 | O2 |
| Ref. 20 | Old Rifle, CO | 0.17 ± 0.1 | 288/0.30 | Geobacter metallireducens | 20 | — | 6 | Acetate 3 mM | 20 | O2/NO3− |
| Ref. 21 | Gravel from Dankritz, Germany | — | 4800/7 | — | 55 | 0.3 | 12 | Lactate 2.8 mM | 6 | O2 |
Sulfate is a common component in groundwater at both Oak Ridge FRC site and Rifle UMTRA site.4,5,24,25 We hypothesized that the addition of supplemental organic carbon would enhance the bioreduction of sulfate. Sulfide produced by sulfate reducing activity would protect biogenic uraninite from remobilization under oxidizing conditions. To test our hypothesis, we conducted column experiments using saprolite from Oak Ridge FRC site. The weathered saprolite at the Oak Ridge FRC is highly fractured and the hydraulic residence time at the Oak Ridge FRC has been predicted to range from 20 to 50 days.26 To simulate the hydrology of Oak Ridge FRC site, we designed our experiments to operate at an exceptionally slow flow rate (1 mL per day) resulting in hydraulic residence time that closely approximated those of the field site (20 days). Ethanol was selected for field studies because it supported faster U(VI) reduction than acetate or lactate8 and, therefore, was selected as the supplemental organic carbon in this study. Ethanol at concentrations of 1 and 10 mM (0.02 mmol per kg per day and 0.2 mmol per kg per day) were used to evaluate the role of supplemental organic carbon on the bioreduction of U(VI) and subsequent reoxidation of biogenic U(IV). The bioreduction phase of the experiment was conducted for 620 days (flow rate 1 mL per day, 31 pore volumes) and then followed reoxidation by oxygen and nitrate for 110 days (flow rate 33 mL per day, 182 pore volumes). Batch reoxidation experiments were also conducted to simulate bulk air reoxidation condition.
Experimental
Column construction and bioreduction experiments
Uranium-contaminated sediment was collected from a depth of 5 to 7 m below ground surface from a series of well borings within Area 2 of the FRC. Detailed descriptions of the sediment and groundwater characteristics of Area 2 have been reported in several other studies.9,10 Characterization of sediments by Mössbauer spectrometry showed that this sediment contained significant quantities of goethite (ca. 64.8% of total Fe), Fe-bearing clay minerals (ca. 35.2% of total Fe) (ESI Fig. S1†). Approximately 14.2% of the Fe-bearing clay minerals Fe was as Fe(II) (Table 2). Columns were constructed and operated as previously described27 using gently crushed FRC sediments.| Hydrofluoric acid extractable Fe (μmol Fe/g) | 820 |
| Oxide Fe(III) (%) | 64.8 |
| Silicate [Fe(III) + Fe(II)] (%) | 35.2 Fe(II)/total Fe = 0.14 |
| Surface area (m2 g−1) | 32.2 |
| Organic carbon content (%) | 0.49 |
Borosilicate glass chromatography columns (Omnifit; 25 mm dia, 150 mm length) fitted with PTFE end caps (one fixed, one adjustable-length) were “wet packed” with sediment such that the water column height above the sediment–water interface was constant when incremental masses of sediment were added to the column. Four sediment columns were constructed to provide duplicates for the two ethanol concentrations tested. Fifty g sediment was added to each column. The adjustable end caps were used to consolidate and secure the sediments and yielded an average packed bed length of 10 cm. Artificial groundwater (AGW) was used as the mobile phase for columns and was based on groundwater collected from well GW835 at FRC Area 2 and modified to include piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES) buffer. AGW included 10 mM PIPES, 5.0 mM NaHCO3, 4.1 mM CaCl2, 1.1 mM MgCl2, 0.16 mM KCl, 1.0 mM Na2SO4, 1.0 mM NaNO3, 2.0 μM uranyl(VI) acetate, 0.10 mM NH4Cl and 0.01 mM KH2PO4. Ethanol was added to the AGW at concentrations of 1 or 10 mM. The AGW pH was adjusted to 6.5 with HCl and NaOH. AGW was autoclaved, then purged and maintained under an 85% N2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15% CO2 headspace at all times. Columns were attached to the different influent solutions using individual cartridges connected to a single peristaltic pump head and adjusted to deliver AGW up-flow at an average flow rate of 1 mL per day. Hydraulic residence time of the columns were determined from 3H breakthrough curves at the start of the bioreduction period and from Br− breakthrough curves at the start of the reoxidation period. The average column pore volume (PV) was 20 mL (equivalent to a porosity of 40%, the calculation of PV is provided in ESI†).
15% CO2 headspace at all times. Columns were attached to the different influent solutions using individual cartridges connected to a single peristaltic pump head and adjusted to deliver AGW up-flow at an average flow rate of 1 mL per day. Hydraulic residence time of the columns were determined from 3H breakthrough curves at the start of the bioreduction period and from Br− breakthrough curves at the start of the reoxidation period. The average column pore volume (PV) was 20 mL (equivalent to a porosity of 40%, the calculation of PV is provided in ESI†).
Column effluents were periodically collected, filtered (0.2 μm) and concentrations of U(VI), NO3− and SO42− were measured (described below). Effluent pH was periodically measured using an in-line microelectrode. One replicate column for each ethanol concentration was destructively sampled after 620 days. Bioreduced sediment samples were analyzed for total reduced inorganic sulfur (TRIS) and acid volatile sulfide (AVS) as described below.
Column reoxidation experiments
At the end of the bioreduction period, the column influent solutions were changed to a single common AGW influent solution that excluded ethanol, nitrate, U(VI), and sulfate. The solution was purged and maintained under a 65% N2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15% CO2
15% CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20% O2 gas mix, and was pumped up-flow through the columns at a flow rate of 33 mL per day for 46 days (1.65 PV per day). After no U(VI) was detected in the column effluents during this period, 1.0 mM NaNO3 was added to the column influent for an additional 64 days (still at 1.65 PV per day). Column effluents were collected and analyzed for U(VI), NO3−, and SO42− as described below.
20% O2 gas mix, and was pumped up-flow through the columns at a flow rate of 33 mL per day for 46 days (1.65 PV per day). After no U(VI) was detected in the column effluents during this period, 1.0 mM NaNO3 was added to the column influent for an additional 64 days (still at 1.65 PV per day). Column effluents were collected and analyzed for U(VI), NO3−, and SO42− as described below.
Batch reoxidation experiments
Bioreduced sediments were collected during column deconstruction and suspended in anaerobic AGW (2 g sediment/25 mL AGW), and were incubated statically under a headspace of 85% air![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15% CO2.16 O2-free control incubations were maintained under a headspace of 85% N2
15% CO2.16 O2-free control incubations were maintained under a headspace of 85% N2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15% CO2. Samples were periodically removed with sterile needle and syringe (in anoxic chamber) and NaHCO3-extractable U(VI) was measured as described below.
15% CO2. Samples were periodically removed with sterile needle and syringe (in anoxic chamber) and NaHCO3-extractable U(VI) was measured as described below.
Analytical techniques
Solid-associated U(VI) was extracted from sediments using anoxic 1 M NaHCO3 (pH 8.4) as described by Elias et al.28 Soluble U(VI) and 1 M NaHCO3 (pH 8.4) extractable U(VI) were quantified by kinetic phosphorescence analysis on a KPA-11 (ChemChek Instruments, Richland, WA).29 Acid volatile sulfide (AVS) and total reduced inorganic sulfur (TRIS) were extracted30 and quantified colorimetrically.31 Anions (including NO3−, NO2−, SO42− and Br−) were quantified by ion chromatography on a Dionex 100 system fitted with an AS4A column with conductivity detection (Dionex Corp., Sunnyvale, CA, USA). Sediment organic carbon content was determined by high temperature combustion method by Huffman Laboratories, Inc. (Golden, CO).The structure of microbial community was also characterized based on 16S rRNA genes analysis. The details of DNA isolation, amplification, cloning and sequencing are provided in the ESI.†
Results and discussion
Slow-flow bioreduction conditions
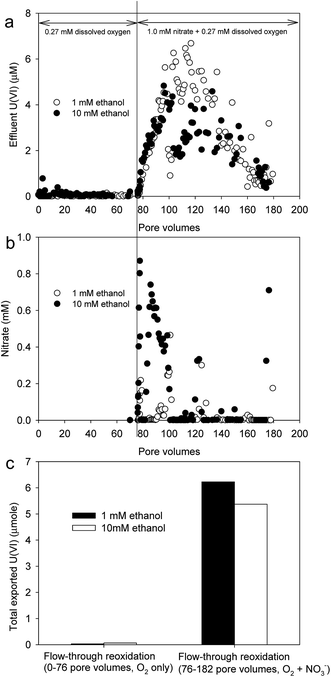
Slow-flow rate was maintained in this study. This slow-flow condition was selected to correspond to long residence times within the micropore domain of the weathered saprolite where the majority of U mass is expected to reside.26 Under this hydraulic condition, the effluent U(VI) concentration dropped rapidly within the first pore volume (i.e., 20 days). After the first pore volume (PV), the effluent U(VI) concentrations remained very low, often near the detection limit of the KPA (0.5 nM), for the remainder of the experiment (620 days, 31 PVs). Over the final 30 PVs, the average effluent U(VI) concentrations from the columns supplied 1 or 10 mM ethanol were 0.024 ± 0.064 μM (n = 153) and 0.025 ± 0.066 μM (n = 185), respectively. The influent ethanol concentration (1 or 10 mM) had almost no effect on the transport of U(VI) out of these columns (Fig. 1). The relatively small effect of a 10-fold increase in ethanol was likely due to that both ethanol concentrations used (1 or 10 mM) were excess of the amount of electron donor necessary to support complete bioreduction of uranyl(VI) (2.0 μM). Additionally, the relatively high organic carbon content of these sediments (0.49%) was higher than that reported in previous studies (0.17%),17,20 and would have been sufficient to support complete U(VI) reduction regardless of exogenous electron donor addition. A previous study illustrated that when sufficient natural organic carbon is available in sediments, additional electron donor had no effect on U(VI) reduction.7 | ||
| Fig. 1 Effluent concentrations of U(VI) as a function of time during the period of anoxic, ethanol-amended AGW addition (620 days, 31 PVs). | ||
The influent ethanol concentration also had almost no effect on the consumption of nitrate and sulfate. Nitrate and sulfate dropped rapidly within the first pore volume and remained low until the end of the experiment (ESI Fig. S2†). In both the 1 and 10 mM ethanol columns, effluent NO3− concentrations dropped to less than 0.02 mM within 2 days and then reached steady-state (0.001 ± 0.002 mM, n = 372). In both 1 and 10 mM ethanol columns, effluent SO42− concentrations dropped to less than 0.1 mM within 50 days and then reached steady-state (0.032 ± 0.110 mM, n = 349). Effluent aqueous Fe(II) concentrations averaged around 40 μM from 100 to 400 days and then declined to approximately 5 μM from 400 to 650 days (data not shown). Biogenic Fe(II) was likely sorbed to mineral surfaces or retained in the column as iron sulfides. Effluent Fe(II) concentrations, therefore, did not adequately reflect the onset and duration of Fe(III)-reducing conditions.
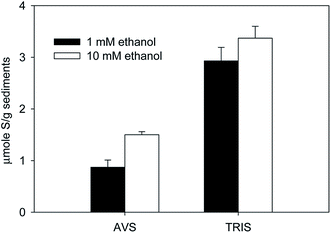
Sediment extractions after the 620-day bioreduction period (31 PV) also revealed the extent sulfate reduction. Concentrations of acid volatile sulfides (AVS) and total reduced inorganic sulfide (TRIS) were both greater in the columns supplied with 10 mM ethanol (Fig. 2). Based on 16S rRNA gene sequences from sediment samples collected from the columns at the end of the bioreduction period, the microbial communities differed depending on the influent ethanol concentration (Fig. 3). Compared to the columns supplied 1 mM ethanol, the percent of proteobacteria increased while the percent of firmicutes decreased in the columns supplied 10 mM ethanol (Fig. 3). These results indicate that different extents of ethanol addition induced shifts in microbial communities, and likely changes in microbial activities, as indicated in the differences in sulfide accumulation.
 | ||
| Fig. 2 Acid volatile sulfide (AVS) and total reduced inorganic sulfur (TRIS) from sacrificed columns after 620 days (31 PVs) of operation receiving 1 mM or 10 mM ethanol in AGW. | ||
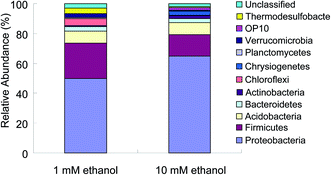 | ||
| Fig. 3 Microbial community characterization from sacrificed columns after 620 days of operation receiving 1 mM or 10 mM ethanol in AGW. | ||
Fast-flow reoxidation conditions
The stability of reduced U(IV) when/if it is exposed to oxidizing conditions is a major issue related to in situ U immobilization. A higher electron donor concentration may promote more rapid U(VI) reduction that yields finer-grained U(IV) precipitates that are more prone to oxidative re-dissolution.16 A higher electron donor concentration may yield higher dissolved carbonate concentrations that may increase the solubility of U(IV) or U(VI).32 Alternatively, a higher electron donor concentration may yield higher concentrations of reduced species that effectively protect the reduced U(IV). In other words, reduced S species may be preferentially and sacrificially oxidized by intruding oxidants, preserving, at least temporarily, the reduced U(IV).In order to test the stability of reduced U(IV) in these columns, “aerated” (65% N2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15% CO2
15% CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20% O2 gas mix) AGW (with no ethanol, nitrate or sulfate) was pumped through the columns at a relatively rapid rate. During the bioreduction period the column flow rate was 0.05 PV per day−1 but during the reoxidation period the flow rate was increased to 1.65 PV per day−1 to simulate conditions associated with fast oxygen intrusion. Initially, dissolved oxygen was provided as the sole oxidant (0.27 mM influent concentration) and did not mobilize U from the columns (Fig. 4). During the first 76 PVs with aerated AGW, effluent dissolved U(VI) concentrations were nearly always less than 0.06 μM from both the 1 and 10 mM ethanol columns. The total mass of U(VI) exported from the columns (in moles) during the flow-through reoxidation experiments was calculated as:
20% O2 gas mix) AGW (with no ethanol, nitrate or sulfate) was pumped through the columns at a relatively rapid rate. During the bioreduction period the column flow rate was 0.05 PV per day−1 but during the reoxidation period the flow rate was increased to 1.65 PV per day−1 to simulate conditions associated with fast oxygen intrusion. Initially, dissolved oxygen was provided as the sole oxidant (0.27 mM influent concentration) and did not mobilize U from the columns (Fig. 4). During the first 76 PVs with aerated AGW, effluent dissolved U(VI) concentrations were nearly always less than 0.06 μM from both the 1 and 10 mM ethanol columns. The total mass of U(VI) exported from the columns (in moles) during the flow-through reoxidation experiments was calculated as:
| U(VI) exported = ∑[U(VI)]i × ΔVi | (1) |
The addition of nitrate (1.0 mM NaNO3) to the aerated influent dramatically increased the oxidation and export of U from the columns (Fig. 4). The initial rapid detection of nitrate in the column effluents reflected its transport as a conservative tracer of sorts. However, as the nitrate-addition period continued, effluent nitrate concentrations decreased, indicative of biological nitrate reduction occurring in the columns (Fig. 4b). The reoxidation of U(IV) under these conditions could have been driven by “direct” biological nitrate-dependent U(IV) oxidation or by “indirect” biological nitrate-dependent Fe(II) oxidation. In the direct route, microbes couple nitrate reduction to U(IV) oxidation.33 In the indirect route, the production of biogenic Fe(III) can catalyze the oxidative dissolution of uraninite and/or the oxidative dissolution of pyrite.34–36 The oxidation of Fe sulfides would remove any “redox protection” that these minerals may have provided U(IV). During this nitrate-amended reoxidation period (76–182 PVs) a total of 6.23 and 5.37 μmoles U(VI), respectively, were exported from the 1 and 10 mM ethanol columns.
Batch reoxidation conditions
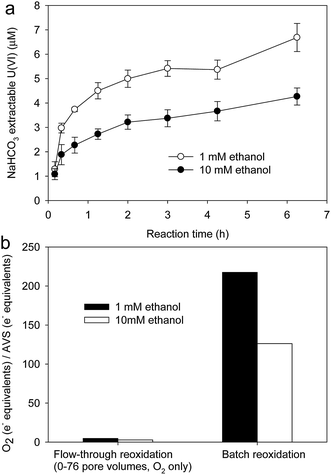
Oxygen is known to be an effective oxidant of uraninite, yet the addition of oxygen did not mobilize U from the flow-through columns (Fig. 4a). Because of the relatively low solubility of oxygen and the high sediment mass-to-water volume ratio in the columns, the delivery of oxygen to U(IV) may have been limited, thus minimizing the observable extent of U(IV) oxidation. Therefore, batch experiments were conducted at a much lower sediment mass-to-water volume ratio to further examine the effect of oxygen on U in these sediments. Under these conditions, U(VI) was immediately and rapidly oxidized (Fig. 5).We speculate that metal sulfides effectively consumed influent oxygen in the flow-through reoxidation experiments but could not protect U(IV) in the batch experiments because of the much higher oxygen to sulfide ratios established in the two experimental systems. In the flow-through experiments, the total oxidizing equivalents from the influent dissolved oxygen was calculated as:
| O2 imported = ∑4 × [O2]i × ΔVi | (2) |
| O2 batch = ∑4 × [O2]V | (3) |
| S in sediments = 8[AVS] × Msediment | (4) |
The rate of U(IV) oxidation in incubations containing sediments from the 1 mM ethanol column was faster than that observed in incubations that contained sediments from the 10 mM ethanol column (Fig. 5a). The total reoxidized U(VI) in 1 mM ethanol column was 0.17 μmole, which was 1.5 times higher than that in 10 mM ethanol columns (0.11 μmole). Our results showed that addition of a higher concentration of ethanol induced conditions that protected biogenic U(VI) from oxidation by oxygen compared to lower concentration of ethanol. Previous studies showed that biogenic FeS could retard U(IV) reoxidation from oxidation by oxygen.20,21 A recent study has confirmed that FeS is effective oxygen scavenging, which inhibited U(IV) from oxidation by oxygen.23 Given that AVS in 10 mM ethanol columns (1.50 μmol g−1, Fig. 2) was higher than that in 1 mM ethanol columns (0.87 μmol g−1, Fig. 2), we speculated that higher concentration of supplemental organic carbon enhanced the bioreduction extent of sulfate, which further protected biogenic U(IV) from oxidation by oxygen.
Implications for the bioremediation of uranium contaminant
Previous work showed that excessive addition of supplemental organic carbon (1.4 mmol per kg per day lactate or acetate) could induce release of aqueous U(VI) even under anoxic/U(VI) reducing conditions. Such U(VI) solubilization under reducing conditions is due to the formation of soluble U(VI)-carbonates that result from organic carbon mineralization.15 Our results from experiments that did not include organic carbon addition rates as high as those of Wan et al.32 (0.2 mmol per kg per day versus 1.4 mmol per kg per day) demonstrate an intermediate supplemental organic carbon addition rate did not increase U(VI) concentration under U(VI) reducing conditions. While no enhancement of U(VI) reduction was observed with greater additions of organic carbon, higher organic carbon addition appeared to induce conditions in the sediments in which U(IV) reoxidation was minimized upon introduction of oxidants. The limited U(IV) remobilization may be partially attributable to the higher sulfide content of the sediments, which served to “protect” U(IV) from reoxidation.20,21,23 This protection is more efficient for oxygen-supported oxidation than nitrate oxidation. Our results highlight that the stability of biogenic U(IV) also should be considered when design supplemental organic carbon supply rate in the presence of sulfate in situ remediation of uranium contaminated aquifers. An intermediate supplemental organic carbon supply rate could promote sulfate reduction and minimize U(IV) remobilization upon intrusion of oxidants.Conclusions
This study investigated effects of supplemental organic carbon (ethanol) addition on the long-term reduction and subsequent reoxidation of uranium. Results showed that a higher concentration of ethanol (10 mM) supported more extensive sulfate reduction compared to lower ethanol amendment (1 mM), which led to greater retention of sulfide in the columns as AVS (e.g. FeS phases). Both 1 and 10 mM ethanol fed columns were resistant to reoxidation in the presence of small amount of oxygen in flow-through reoxidation experiments (O2 to AVS ratio was 2.7 to 4.7). However, in the presence of bulk oxygen in batch reoxidation experiments (O2 to AVS ratios of 126 and 218), sediments in columns that received a higher concentration of ethanol (10 mM) exhibited less U(IV) reoxidation. Similar results were observed where nitrate was used as an oxidant. AVS (e.g. FeS phases) in 10 mM ethanol columns was higher than that in 1 mM ethanol columns (Fig. 2), and was speculated as the main factor to protected biogenic U(IV) from reoxidation.Acknowledgements
We thank Dr Christopher A. Gorski (The Pennsylvania State University) for the collection of Mössbauer spectra and modeling. This work was supported by the Natural and Accelerated Bioremediation Research (NABIR) Program, Office of Biological and Environmental Research (BER), Office of Energy Research, U.S. Department of Energy (DOE) grant no. DE-FG02-01ER631180 to The Pennsylvania State University.References
- U.S. Department of Energy, Bioremediation of metals and radionuclides: what it is and how it works, 2nd edn, 2003 Search PubMed.
- K. T. Finneran, R. T. Anderson, K. P. Nevin and D. R. Lovley, Soil Sediment Contam., 2002, 11, 339–357 CrossRef CAS.
- S. R. Mohanty, B. Kollah, D. B. Hedrick, A. D. Peacock, R. K. Kukkadapu and E. E. Roden, Environ. Sci. Technol., 2008, 42, 4384–4390 CrossRef CAS.
- R. T. Anderson, H. A. Vrionis, I. Ortiz-Bernad, C. T. Resch, P. E. Long, R. Dayvault, K. Karp, S. Marutzky, D. R. Metzler, A. Peacock, D. C. White, M. Lowe and D. R. Lovley, Appl. Environ. Microbiol., 2003, 69, 5884–5891 CrossRef CAS.
- J. D. Istok, J. M. Senko, L. R. Krumholz, D. Watson, M. A. Bogle, A. Peacock, Y. J. Chang and D. C. White, Environ. Sci. Technol., 2004, 38, 468–475 CrossRef CAS.
- W. M. Wu, J. Carley, J. Luo, M. A. Ginder-Vogel, E. Cardenas, M. B. Leigh, C. C. Hwang, S. D. Kelly, C. M. Ruan, L. Y. Wu, J. Van Nostrand, T. Gentry, K. Lowe, T. Mehlhorn, S. Carroll, W. S. Luo, M. W. Fields, B. H. Gu, D. Watson, K. M. Kemner, T. Marsh, J. Tiedje, J. Z. Zhou, S. Fendorf, P. K. Kitanidis, P. M. Jardine and C. S. Criddle, Environ. Sci. Technol., 2007, 41, 5716–5723 CrossRef CAS.
- J. M. Senko, J. D. Istok, J. M. Suflita and L. R. Krumholz, Environ. Sci. Technol., 2002, 36, 1491–1496 CrossRef CAS.
- W. M. Wu, J. Carley, T. Gentry, M. A. Ginder-Vogel, M. Fienen, T. Mehlhorn, H. Yan, S. Caroll, M. N. Pace, J. Nyman, J. Luo, M. E. Gentile, M. W. Fields, R. F. Hickey, B. H. Gu, D. Watson, O. A. Cirpka, J. Z. Zhou, S. Fendorf, P. K. Kitanidis, P. M. Jardine and C. S. Criddle, Environ. Sci. Technol., 2006, 40, 3986–3995 CrossRef CAS.
- J. W. Moon, Y. Roh, T. J. Phelps, D. H. Phillips, D. B. Watson, Y. J. Kim and S. C. Brooks, J. Environ. Qual., 2006, 35, 1731–1741 CrossRef CAS PubMed.
- F. Zhang, W. M. Wu, J. C. Parker, T. Mehlhorn, S. D. Kelly, K. M. Kemner, G. X. Zhang, C. Schadt, S. C. Brooks, C. S. Criddle, D. B. Watson and P. M. Jardine, J. Hazard. Mater., 2010, 183, 482–489 CrossRef CAS PubMed.
- D. R. Lovley, J. P. Phillips, Y. A. Gorby and E. R. Landa, Nature, 1991, 350, 413–416 CrossRef CAS.
- D. R. Lovley, E. E. Roden, E. J. Phillips and J. C. Woodward, Mar. Geol., 1993, 113, 41–53 CrossRef CAS.
- E. L. Brodie, T. Z. DeSantis, D. C. Joyner, S. M. Baek, J. T. Larsen, G. L. Andersen, T. C. Hazen, P. M. Richardson, D. J. Herman, T. K. Tokunaga, J. M. M. Wan and M. K. Firestone, Appl. Environ. Microbiol., 2006, 72, 6288–6298 CrossRef CAS PubMed.
- M. Y. Xu, W. M. Wu, L. Y. Wu, Z. L. He, J. D. Van Nostrand, Y. Deng, J. Luo, J. Carley, M. Ginder-Vogel, T. J. Gentry, B. H. Gu, D. Watson, P. M. Jardine, T. L. Marsh, J. M. Tiedje, T. Hazen, C. S. Criddle and J. Z. Zhou, ISME J., 2010, 4, 1060–1070 CrossRef PubMed.
- T. K. Tokunaga, J. M. Wan, Y. M. Kim, R. A. Daly, E. L. Brodie, T. C. Hazen, D. Herman and M. K. Firestone, Environ. Sci. Technol., 2008, 42, 8901–8907 CrossRef CAS.
- J. M. Senko, S. D. Kelly, A. C. Dohnalkova, J. T. McDonough, K. M. Kemner and W. D. Burgos, Geochim. Cosmochim. Acta, 2007, 71, 4644–4654 CrossRef CAS PubMed.
- H. S. Moon, J. Komlos and P. R. Jaffe, Environ. Sci. Technol., 2007, 41, 4587–4592 CrossRef CAS.
- J. Komlos, A. Peacock, R. K. Kukkadapu and P. R. Jaffe, Geochim. Cosmochim. Acta, 2008, 72, 3603–3615 CrossRef CAS PubMed.
- J. Komlos, B. Mishra, A. Lanzirotti, S. C. B. Myneni and P. R. Jaffe, J. Environ. Eng., 2008, 134, 78–86 CrossRef CAS.
- H. S. Moon, J. Komlos and P. R. Jaffe, J. Contam. Hydrol., 2009, 105, 18–27 CrossRef CAS PubMed.
- F. Dullies, W. Lutze, W. L. Gong and H. E. Nuttall, Sci. Total Environ., 2010, 408, 6260–6271 CrossRef CAS PubMed.
- J. Carpenter, Y. Q. Bi and K. F. Hayes, Environ. Sci. Technol., 2015, 49, 1078–1085 CrossRef CAS PubMed.
- Y. Q. Bi and K. F. Hayes, Environ. Sci. Technol., 2014, 48, 632–640 CrossRef CAS PubMed.
- D. B. Watson, J. E. Kostka, M. W. Fields and P. M. Jardine, The oak ridge field research center conceptual model, NABIR Field Research Center, Oak Ridge, Tennessee, 2004.
- K. M. Campbell, H. Veeramani, K. U. Urich, L. Y. Blue, D. E. Giammar, R. Bernier-Latmani, J. E. Stubbs, E. Suvorova, S. Yabusaki, J. S. Lezama-Pacheco, A. Mehta, P. E. Long and J. R. Bargar, Environ. Sci. Technol., 2011, 45, 8748–8754 CrossRef CAS PubMed.
- E. E. Roden and T. D. Scheibe, Chemosphere, 2005, 59, 617–628 CrossRef CAS PubMed.
- M. L. Minyard and W. D. Burgos, Environ. Sci. Technol., 2007, 41, 1218–1224 CrossRef CAS.
- D. A. Elias, J. M. Senko and L. R. Krumholz, J. Microbiol. Methods, 2003, 53, 343–353 CrossRef CAS.
- R. Brina and A. G. Miller, Anal. Chem., 1992, 64, 1413–1418 CrossRef CAS.
- G. A. Ulrich, L. R. Krumholz and J. M. Suflita, Appl. Environ. Microbiol., 1997, 63, 1627–1630 CAS.
- J.D. Cline, Limnol. Oceanogr., 1969, 14, 454–458 CrossRef CAS.
- J. M. Wan, T. K. Tokunaga, Y. M. Kim, E. Brodie, R. Daly, T. C. Hazen and M. K. Firestone, Environ. Sci. Technol., 2008, 42, 7573–7579 CrossRef CAS.
- K. T. Finneran, M. E. Housewright and D. R. Lovley, Environ. Microbiol., 2002, 4, 510–516 CrossRef CAS.
- J. M. Senko, Y. Mohamed, T. A. Dewers and L. R. Krumholz, Environ. Sci. Technol., 2005, 39, 2529–2536 CrossRef CAS.
- M. Ginder-Vogel, C. S. Criddle and S. Fendorf, Environ. Sci. Technol., 2006, 40, 3544–3550 CrossRef CAS.
- J. M. Senko, J. M. Suflita and L. R. Krumholz, Geomicrobiol. J., 2005, 22, 371–378 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra02550h |
| This journal is © The Royal Society of Chemistry 2015 |