Steric group enforced aromatic cyclic trimer conformer in tripodal molecules†
Shankar Deval Sathiyashivana,
Bhaskaran Shankarb,
Palanisamy Rajakannua,
Pratap Vishnoic,
Dhanraj T. Masram*a and
Malaichamy Sathiyendiran*ab
aDepartment of Chemistry, University of Delhi, India. E-mail: dtmasram@chemistry.du.ac.in; mssc@uohyd.ernet.in
bSchool of Chemistry, University of Hyderabad, India
cDepartment of Chemistry, Indian Institute of Technology Bombay, India
First published on 26th August 2015
Abstract
A family of tripodal molecules (1–6) with/without steric ethyl groups at the central benzene scaffold and with furan/thiophene/pyridyl group at the 2-position of the benzimidazolyl unit was synthesised. Compounds 1–6 were characterized by elemental analysis and NMR spectroscopy. Compounds 1, 3, and 5 were further characterized by single crystal X-ray diffraction analysis. The molecular structures of 1 and 4 were optimized using density functional theory (DFT) calculations. X-ray and 1H NMR studies reveal that the introduction of three ethyl groups into a central benzene scaffold of furan/thiophene/pyridyl substituted benzimidazolyl based tripodal molecules enhances the edge-to-face C–H⋯π interactions, thereby favouring the aromatic cyclic trimer motif, in solution and the solid state. The unsubstituted central benzene scaffold allows the furan/thiophene substituted benzimidazolyl units in the tripodal molecules to move freely thereby weakening the edge-to-face C–H⋯π interactions between the aromatic cyclic trimer motif. Molecular modelling calculations indicate that the energy minimized structures of the tripodal molecules adopt a symmetric cyclic aromatic motif conformation.
Introduction
Non-covalent aromatic motifs between heterocyclic units/phenylene units via the face-to-face π⋯π, and the edge-to-face C–H⋯π interactions make important contributions to biomolecular structures and functions.1–3 Among several man-made molecules with aromatic motifs including dimers, trimers (ladder and cyclic clusters), tetramers, pentamers and higher order clusters, discrete molecules with aromatic cyclic trimer motif structures (Fig. 1), in solution or even in the solid state are very limited.4–6 Due to the presence of aromatic cyclic trimer motif in half of all proteins and such motif is thought of as the basic building unit of higher order clusters, efforts are being directed toward the synthesis of molecule with aromatic cyclic trimer motif.4–6Recently, two tripodal molecules (1,3,5-tri(2-(furan-2-yl)benzimidazol-1-ylmethyl)-2,4,6-trimethylbenzene (I), and 1,3,5-tri(2-(thiophen-2-yl)benzimidazol-1-ylmethyl)-2,4,6-tri methyl benzene (II) (Fig. 2)), possessing the benzene cyclic trimer motif, which is stabilized by the edge-to-face C–H⋯π interactions, both in solution and solid state were reported by us.6 The studies on I, and II indicate that the heterocyclic moiety at the 2-position of the benzimidazolyl (bim) enforces the three benzimidazolyl units to form the benzene cyclic trimer.6 In order to explore further and make library of discrete aromatic cyclic trimer, molecules I and II were sterically modified both at the central benzene scaffold and at the 2-position of the benzimidazolyl unit.
Herein, six new tripodal molecules 1,3,5-tri(2-(furan-2-yl)benzimidazol-1-ylmethyl)-2,4,6-triethylbenzene (1), 1,3,5-tri(2-(furan-2-yl)benzimidazol-1-ylmethyl)-benzene (2), 1,3,5-tri(2-(thiophen-2-yl)benzimidazol-1-ylmethyl)-2,4,6-triethyl-benzene (3), 1,3,5-tri(2-(thiophen-2-yl)benzimidazol-1-ylmethyl)-benzene (4), 1,3,5-tri(2-(pyridyl-2-yl)benzimidazol-1-ylmethyl)-2,4,6-triethylbenzene (5), and 1,3,5-tri(2-(phenyl)benzimidazol-1-ylmethyl)-2,4,6-triethylbenzene (6) are reported (Fig. 2). Molecules 1–6 were characterized by elemental analysis, nuclear magnetic resonance (NMR) spectroscopy. The molecular structures of 1, 3 and 5 were further confirmed using single-crystal X-ray diffraction (XRD) analysis. The molecular structures of 1 and 4 were optimized using density functional theory (DFT) calculations.
Results and discussion
Preparations
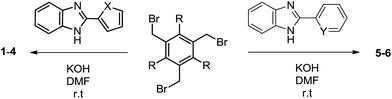
Molecules 1–6 were prepared by the treatment of substituted benzimidazole (2-(furan-2-yl)-1H-benzimidazole (H-fbim) for 1-2; 2-(thiophen-2-yl)-1H-benzimidazole (H-tbim) for 3-4; 2-(pyridyl-2-yl)-1H-benzimidazole (H-pbim) for 5; 2-(phenyl)benzimidazole (H-phbim) for 6) and 1,3,5-tri(bromomethyl)-2,4,6-triethylbenzene or 1,3,5-tri(bromomethyl)-benzene and KOH in DMF (Scheme 1).6–9 All molecules are air and moisture stable and soluble in organic solvents.Single crystal X-ray diffraction studies
The results of single-crystal X-ray studies showed that 1, 3 and 5 adopt a syn-conformation i.e., ababab-type with three benzimidazolyl (bim) units on one side of the central benzene scaffold and three furan/thiophene/pyridyl and three methyl groups on the other side (Fig. 3, 4 and S1 in ESI†). Three benzene of bim rings are arranged in an almost the edge-to-face arrangement with the benzene edge directed over the centre of mass (COM) benzene ring of an adjacent benzene unit of bim. Three benzene of the bim contact each other through the C–H⋯π interactions (Table 1).1 The distances between the COM of the benzene ring of bim units and the dihedral angles between the bim units are within the range for the symmetrical benzene cyclic trimer motif.1 | ||
| Fig. 4 Molecular structure of 5 (left: H atoms are removed). Cyclic aromatic trimer motif in 5 (right: H atoms and other units are omitted). C = gray, N = blue. | ||
| 1 | 1a | 3 | 5 | |
|---|---|---|---|---|
| rab | 5.13 | 5.74 | 5.09 | 5.30 |
| rbc | 5.19 | 5.77 | 5.40 | 5.07 |
| rac | 5.06 | 5.76 | 5.43 | 4.97 |
| τab | 64 | 60 | 65 | 56 |
| τbc | 54 | 59 | 59 | 62 |
| τca | 61 | 60 | 55 | 62 |
The major difference between 1 and 3 is in the orientation of five-member aromatic ring (furan/thiophene). The bim and furan unit in 1 are almost planar. The oxygen atom of furan ring is directed toward the methylene unit (furan-CH2-central benzene), and contacts with the CH2 through the hydrogen bonding interaction. The sulfur atoms are directed away from each other and the centre of the molecule in 3 similar to the thiophene arrangement found in II.6 Each molecule in 1 interacts with three neighbouring molecules using three fbim units. The intermolecular offset face-to-face π⋯π interactions were found between the anti-cofacially arranged fbim units in 1 (Fig. S1 in ESI†).
1H NMR studies
The 1H NMR spectrum of 1 in d6-DMSO (Fig. 5) displayed well-separated, and a single set of signals for furan protons (H3′–H5′) and H4 and H5 protons of benzimidazolyl (bim). The H6 and H7 protons of benzene of bim are merged together in 1. A single sharp peak at δ 5.65 ppm corresponds to the methylene (–CH2–) protons. Among the furan protons, the H3′ and H4′ were slightly shifted downfield in 1 compared to the free fbim. No significant shift was observed for the H5′ proton. The H4 and H5 protons of bim were slightly upfield shifted, whereas the H6 and H7 were remarkably upfield shifted in 1 compared to the free fbim. A single set of chemical resonances for all protons of 1, revealing the presence of a single conformer, preferably syn-conformer or the presence of syn-conformers (c and d in Fig. 6), that undergo a rapid equilibrium in solution on the NMR time scale.6,10 | ||
| Fig. 6 Various possible conformers (a–d) for tripodal ligands due to different orientation of benzimidazolyl units. Ball represents substituted group on benzimidazolyl unit.6 | ||
From the X-ray structure of 1, upfield signals for the H6 and H7 due to the edge-to-face C–H⋯π interactions between three benzene units of bim would be expected. A similar 1H NMR pattern for H6 and H7 was also observed in the case of methyl substituted tripodal molecule I.6 The result supports that the major conformer in solution retain the same structure as that found in the solid state. Other possibility is the presence of a mixture of syn-conformers (c and d in Fig. 6), which undergo a rapid equilibrium on the NMR time scale.6 It is important to compare the chemical resonances of H6 and H7 in the ethyl substituted 1 and the methyl substituted I, because other units are similar in both the molecules. The upfield shift value for H6 is a little higher in 1 than I, which suggested that the steric group on the central benzene of the tripodal molecules influences the orientation of cyclic aromatic trimer.
Molecule 3 in d6-DMSO displays a similar 1H NMR pattern like those of 1 with slight difference of chemical resonance for the H6 and H7 protons (Fig. 7 and S2 in ESI†). Both H6 and H7 signals are started to broaden, while these are as triplet and doublet in the free H-tbim and the methyl substituted tripodal molecule II. However, the H6 proton signal was more upfield shifted similar to 1. The result indicates that molecule 3 also adopts the cyclic aromatic trimer structure like those of I, II, and 1. The broadness of H6 and H7 resonance may be due to either the tbim unit oscillates back and forth slowly or the presence of mixture of syn-conformers, which undergo a slow equilibrium on the NMR time scale. Other protons of the tbim unit in 3 appeared as sharp signals.
In order to ascertain this, a variable temperature 1H NMR experiment was performed for 1 and 3. A single set of well-resolved chemical resonances was observed for both 1 and 3 in d6-DMSO at 50/100 °C. In particular, the H6 and H7 appeared as triplet and doublet with slight downfield shift at high temperature (Fig. 7 and S3†). At low temperature (−55 °C), the H6 and H7 protons of 1 were shifted upfield significantly in relative to room-temperature chemical resonances in CDCl3 (Fig. S4†). No additional peaks were observed at −55 °C for compound 1. Compound 3 showed a single set of peaks for all the protons except H6 and H7 protons (Fig. S5†). Three sets of broad peaks were observed for H6 and H7 protons of 3. The results indicate that three types of syn-conformers exist in solution, which undergo rapid equilibrium at high temperature (50/100 °C), and slow equilibrium at low temperature (−55 °C). Further, the above results reveal that compounds 1 and 3 adopt syn-conformation in d6-DMSO and CDCl3.
The 1H NMR spectra of 5 and 6 in d6-DMSO (Fig. 8 and 9) indicate that these molecules adopt the cyclic aromatic trimer conformer in solution similar to 1 and 3. In addition, the methylene protons (–CH2–) in 5 appeared in the aromatic region as a sharp singlet (δ 6.30 ppm) i.e. downfield shifted compare to the free H-pbim. Molecules I, II, 1–4 and 6 displayed chemical resonance for the methylene around δ ∼ 5.88–5.45 ppm. It suggests that the methylene protons might be involved in the hydrogen bonding interactions with the pyridyl nitrogen donor, thus shifting the methylene resonance to downfield. The known 1,3,5-tri(2-(pyridyl-2-yl)benzimidazol-1-ylmethyl)-2,4,6-trimethylbenzene (III)9 was synthesized using the similar route and studied to compare with 5. The 1H NMR data of III (Fig. 8 middle) clearly indicates that the cyclic aromatic trimer motif present in the solution. This result also supports that the ethyl groups at the central benzene scaffold favour the formation of cyclic aromatic trimer motif.
The 1H NMR spectrum of 2 (middle in the Fig. 5) possessing the unsubstituted benzene centre scaffold in d6-DMSO displayed well-separated, and a single set of signals. The proton pattern of 2 is different from the tripodal molecules with substituted benzene centre scaffold I-II, 1, 3 and 5. The benzimidazolyl protons H4 and H5 are slightly downfield shifted, whereas the H7 and H6 are slightly upfield shifted in 2 compared to the free H-fbim. The protons H4 and H5 are little downfield shifted compare to 1, while H7 and H6 are highly downfield shifted. The single set of signals for all protons with a slight upfield shift for the H7 and H6 protons in 2 in comparison to free fbim may be due to the adoptation of 2 as a syn-conformer possessing the cyclic benzene trimer motif with the very weak edge-to-face C–H⋯π interactions between three benzene units. The furan protons (H3, H4, and H5) appeared in the upfield region in 2 compared to the free H-fbim. These protons H3′, H4′, and H5′ were highly upfield shifted compared to 1. This may be due to either presence of the predominant syn-conformer with the cyclic benzene trimer above to the central benzene scaffold and cyclic thiophene trimer below to the benzene unit in solution or the mixture of two conformers, which undergo rapid equilibrium in the NMR time scale by tilting the furan benzimidazolyl units as shown in Fig. 10. Molecule 4 showed the similar 1H NMR spectral pattern like that of 2 (Fig. S2 in ESI†).
 | ||
| Fig. 10 Two syn-conformers of 2 with the strong edge-to-face C–H⋯π cyclic benzene trimer (left) and without C–H⋯π cyclic benzene trimer interactions. | ||
Computational studies of 1 and 4
A DFT experiment was performed for 1 and 4 to know the global minima of the energy structures. The tripodal molecule possessing ethyl substituent groups on the central benzene scaffold (1) adopt the highly symmetrical, cyclic aromatic trimer conformation with cone-shaped tripodal structure (Fig. S6†).The optimized structure of unsubstituted central benzene scaffold based molecule 4 adopts a propeller like geometry with close to cylindrical shaped structure in which three benzimidazolyl residues are arranged in the edge-to-face arrangement with the benzene edge of benzimidazolyl located over the COM benzene ring of the adjacent benzimidazolyl units (Fig. 11). The average distance between the COM of three aromatic residues is ∼6.18 Å, suggesting that a weak edge-to-face C–H⋯π interaction is present in the cyclic benzene trimer motif.6 However, the cooperative C–H⋯π interactions in the trimer provide stabilization to the syn-conformer. Similarly, all three thiophene units are arranged in a cyclic manner with the COM distance of ∼10.35 Å.
Conclusions
A family of tripodal molecules possessing the cyclic aromatic trimer motif was synthesized using substituted benzimidazole and alkyl-substituted benzene scaffold. In addition, two tripodal molecules without alkyl substitution on the central core were also synthesized and studied. The results clearly confirm that the steric groups furan, thiophene, and pyridine at the 2-position of the benzimidazolyl and alkyl (methyl/ethyl) at the center of benzene scaffold play a role in arranging three benzimidazolyl units. The edge-to-face C–H⋯π interactions between the three benzene units stabilize the cyclic benzene trimer motif both in solution and the solid state. On going from methyl substituted to ethyl substituted at the benzene scaffold, the C–H⋯π interaction among the cyclic benzene trimer is strengthened. On the other hand, the molecule possessing furan/thiophene at the 2-position of benzimidazolyl with unsubstituted benzene scaffold favours syn-conformer structure with very weak/no C–H⋯π interactions between the trimer unit. The work demonstrates that discrete molecules with aromatic cyclic trimer motif arrangement both in solid and solution state can be designed and prepared. This work is part of our research aimed at designing the tripodal molecules with the aromatic cyclic trimer motif.Experimental
General experimental methods
o-Phenylenediamine, 2-thiophenecarboxaldehyde, furan-2-carboxaldehyde, 1,3,5-tris(bromomethyl)-benzene, 1,3,5-tris(bromomethyl)-2,4,6-triethylbenzene (tbteb), mesitylene, 2-pyridyl-2-benzimidazole and dimethylformamide (DMF) were purchased from commercial sources and used as received. 2-Thiophene-2-benzimidazole 2-furan-2-benzimidazole and phenyl-2-benzimidazole were prepared following a similar procedure to that reported earlier (ESI S1–S3†). 1H spectra were recorded on an JEOL ECX 400 NMR and Bruker AVANCEIII 400 and 500 MHz spectrometers operating at 400 MHz. The chemical shifts are reported in parts per million (ppm) relative to residual solvent signal. Melting points for compounds were recorded in BUCHI laboratory equipment-Melting point M-560.Computational details
The geometry optimizations of 1 and 4 were carried out in the DMSO solvent medium using B3LYP method with 6-311+G (d,p)11 basis set using Gaussian 09 program package.12 The initial geometry for 1 was obtained from the X-ray crystal structure coordinates.X-ray crystallography
Intensity data of suitably sized crystals 1 and 3 were collected on a Rigaku Saturn 724+CCD diffractometer for unit cell determination and three dimensional intensity data collection. 800 frames in total were collected at 120 and 150 K, respectively, with the exposure time of 18 s per frame. Data integration, indexing and absorption correction using Crystal clear followed by structure solution using the programs in WinGX module.13a Intensity data of crystal of 5 were collected on Oxford CCD X-ray diffractometer (Xcalibur, Eos, Gemini) equipped with Cu Kα radiation (λ = 1.54184 Å) source. Data reduction was performed using CrysAlisPro 1.171.37.35h (release 09-02-2015 CrysAlis171 NET). The structures were solved by direct methods using SIR 92,13b which revealed the atomic positions, and refined using the SHELXL-2014/7 program (within the WinGX program package).13a,d Non-hydrogen atoms were refined anisotropically. The solvent molecules could not be modelled and hence their contributions to intensities were excluded using SQUEEZE option in PLATON for 1, 3, and 5. The disordered one furan unit in 1 has been successfully modelled using the PART 1 (O3, C22, C45–C47) and PART 2 (O3A, C22A, C45A–C47A) instructions. After refining as a free variable, the site-of-occupancy is almost 0.63 and 0.37, respectively. The nonhydrogen atoms of the furan unit have been constrained or restrained with appropriate instructions such as AFIX43 (O3, C22, C45–C47 in PART 1, and O3A, C22A, C45A–C47A in PART 2), DELU, and SIMU. The detailed crystallographic data's of 1, 3 and 5 are given in ESI (Table S1†).Synthesis and characterization details
1: a mixture of 2-furan-2-benzimidazole (313.9 mg, 1.70 mmol) and KOH (190.8 mg, 3.40 mmol) was stirred in DMF (10 mL) at room temperature for 1 h. 1,3,5-Tri(bromomethyl)-2,4,6-triethylbenzene (250.5 mg, 0.56 mmol) was added to the reaction mixture and continuously allowed to stir for 72 h. The reaction was quenched by adding water (200 mL). The powder was collected by filtration. Yellow colour crystals 1 were obtained from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 chloroform/methanol at room temperature after few days. Yield: 94% (400.6 mg, 0.53 mmol, for C48H42N6O3). Mp: 251–253 °C (dec.). 1H NMR (400 MHz, DMSO-d6): δ 7.99 (s, 3H, H5′), 7.60 (d, 3H, JHH = 7.64 Hz, H4), 7.33 (d, 3H, JHH = 4.04 Hz, H3′), 7.11 (t, 3H, JHH = 7.64 Hz, H5), 6.90–6.77 (m, 3H, H4′), 6.33–6.28 (m, 6H, H6−7), 5.80 (s, 6H, –CH2–), 2.66 (d, 6H, JHH = 7.64 Hz, –CH2–), and 0.76 (s, 9H, –CH3–). HRMS (m/z): [M + H]+ calc for C48H42N6O3, 751.3397; found: 751.3543.
1 chloroform/methanol at room temperature after few days. Yield: 94% (400.6 mg, 0.53 mmol, for C48H42N6O3). Mp: 251–253 °C (dec.). 1H NMR (400 MHz, DMSO-d6): δ 7.99 (s, 3H, H5′), 7.60 (d, 3H, JHH = 7.64 Hz, H4), 7.33 (d, 3H, JHH = 4.04 Hz, H3′), 7.11 (t, 3H, JHH = 7.64 Hz, H5), 6.90–6.77 (m, 3H, H4′), 6.33–6.28 (m, 6H, H6−7), 5.80 (s, 6H, –CH2–), 2.66 (d, 6H, JHH = 7.64 Hz, –CH2–), and 0.76 (s, 9H, –CH3–). HRMS (m/z): [M + H]+ calc for C48H42N6O3, 751.3397; found: 751.3543.
2: a mixture of 2-furan-2-benzimidazole (387.6 mg, 2.104 mmol) and KOH (235.8 mg, 4.20 mmol) was stirred in DMF (10 mL) at room temperature for 1 h. 1,3,5-Tris(bromomethyl)benzene (250.8 mg, 0.702 mmol) was added to the reaction mixture and continuously allowed to stir for 72 h. The reaction was quenched by adding water (200 mL). The pale brown powder was collected by filtration. Yield: 69% (325 mg, 0.487 mmol). 1H NMR (400 MHz, DMSO-d6): δ 7.64 (d, 3H, JHH = 8.4 Hz, H4), 7.56 (s, 3H, H5′), 7.36 (d, 3H, JHH = 8.4 Hz, H7)), 7.24 (t, 3H, JHH = 7.63 Hz, H5), 7.15 (t, 3H, JHH = 7.63 Hz, H6), 6.93 (d, 3H, JHH = 3.05 Hz, H3′), 6.90 (s, 3H, arene), 6.57–6.56 (m, H4′), and 5.56 (s, 6H, –CH2–).
3: a mixture of 2-thiophene-2-benzimidazole (360.5 mg, 1.8 mmol) and KOH (210 mg, 3.742 mmol) was stirred in DMF (10 mL) at room temperature for 1 h. 1,3,5-Tri(bromomethyl)-2,4,6-triethylbenzene (265.8 mg, 0.602 mmol) was added to the reaction mixture and continuously allowed to stir for 72 h. The reaction was quenched by adding water (200 mL). The white powder was collected by filtration and dissolved in hot methanol. Colourless crystals were obtained at room temperature after few days. Yield: 81.19% (391 mg, 0.489 mmol). 1H NMR (400 MHz, DMSO-d6): δ 7.88–7.85 (m, 6H, H4′,5′), 7.60 (d, 3H, JHH = 7.96 Hz, H4), 7.29 (t, 3H, JHH = 4.26 Hz, H3′), 7.12 (t, 3H, JHH = 7.64 Hz, H5), 6.36 (s, 3H, H6), 6.20 (d, 3H, JHH = 7.92 Hz, H7), 5.69 (s, 6H), 2.5 (6H, –CH2–) and 0.65 (s, 9H, H9). HRMS (m/z): [M + H]+ calc for C48H43N6S3, 799.2633; found: 799.2688.
4: a mixture of 2-thiophene-2-benzimidazole (445.2 mg, 2.22 mmol) and KOH (250 mg, 4.44 mmol) was stirred in DMF (10 mL) at room temperature for 1 h. 1,3,5-Tris(bromomethyl)benzene (264.6 mg, 0.741 mmol) was added to the reaction mixture and continuously allowed to stir for 72 h. The reaction was quenched by adding water (200 mL). The pale yellow powder was collected by filtration. Yield: 62% (328 mg, 0.458 mmol). 1H NMR (400 MHz, DMSO-d6): δ 7.68–7.64 (m, 6H, H5′ & H4), 7.33 (d, 3H, JHH = 7.92 Hz, H7), 7.23 (t, 3H, JHH = 7.32 Hz, H5), 7.18–7.15 (m, 6H, H4′ & H6), 6.94–6.91 (m, 3H, H3′), 6.75 (s, 3H, arene), and 5.54 (s, 6H, –CH2–).
5: a mixture of 2-pyridyl-2-benzimidazole (351.9 mg, 1.80 mmol) and KOH (201.7 mg, 3.59 mmol) was stirred in DMF (10 mL) at room temperature for 1 h. 1,3,5-tri(bromomethyl)-2,4,6-triethylbenzene (264.5 mg, 0.59 mmol) was added to the reaction mixture and continuously allowed to stir for 72 h. Colourless needle type crystals of 5 were obtained by crystallization from hot chloroform/methanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) after few days at room temperature. Yield: 87% (409.1 mg, 0.52 mmol). 1H NMR (400 MHz, DMSO-d6): δ 8.76 (d, 3H, JHH = 5.36 Hz, H6′), 8.28 (d, 3H, JHH = 7.64 Hz, H3), 8.03 (d, 3H, JHH = 7.64 Hz, 1.52 Hz, H5′), 7.68 (d, 3H, H4), 7.55–7.52 (m, 3H, H4′), 7.14 (t, 3H, JHH = 7.64 Hz, H5), 6.45 (6H, JHH = 8.36 Hz, H6−7), 6.26 (s, 6H, –CH2–), 2.86–2.82 (m, 6H, –CH2–) and 0.68 (s, 9H, –CH3–). HRMS (m/z): Calc. 784.3876. Found: 784.3854.
1) after few days at room temperature. Yield: 87% (409.1 mg, 0.52 mmol). 1H NMR (400 MHz, DMSO-d6): δ 8.76 (d, 3H, JHH = 5.36 Hz, H6′), 8.28 (d, 3H, JHH = 7.64 Hz, H3), 8.03 (d, 3H, JHH = 7.64 Hz, 1.52 Hz, H5′), 7.68 (d, 3H, H4), 7.55–7.52 (m, 3H, H4′), 7.14 (t, 3H, JHH = 7.64 Hz, H5), 6.45 (6H, JHH = 8.36 Hz, H6−7), 6.26 (s, 6H, –CH2–), 2.86–2.82 (m, 6H, –CH2–) and 0.68 (s, 9H, –CH3–). HRMS (m/z): Calc. 784.3876. Found: 784.3854.
6: a mixture of phenyl-2-benzimidazole (500 mg, 2.57 mmol) and KOH (290.1 mg, 5.17 mmol) was stirred in DMF (10 mL) at room temperature for 1 h. 1,3,5-Tri(bromomethyl)-2,4,6-triethylbenzene (378.3 mg, 0.86 mmol) was added to the reaction mixture and continuously allowed to stir for 72 h. The reaction was quenched by adding water (250 mL). Colourless powder 6 was collected by filtration. Yield: 91% (608.5 mg, 0.78 mmol). 1H NMR (400 MHz, DMSO-d6): δ 7.80 (s, broad, 6H, phenyl), 7.64 (d, 3H, JHH = 8 Hz, H4), 7.56 (s, broad, 9H, phenyl), 7.14 (t, 3H, JHH = 8 Hz, H5), 6.46 (t, JHH = 7.6 Hz, 3H, H6), 6.34 (d, JHH = 8.4 Hz, 3H, H7), 5.45 (s, 6H, –CH2–), 2.44 (d, JHH = 6.8 Hz, 6H, –CH2–) and 0.43 (t, JHH = 6.8 Hz, 9H, –CH3–). HRMS (m/z): [M + H]+ calc for C54H49N6 Calc. 781.4013. Found: 781.4032.
Acknowledgements
We are grateful to Prof. Dr R. V. Krishnakumar, Department of Physics, Thiagarajar College, Madurai-India for his help in solving the structures. We thank Prof. R. Murugavel for the use of his Single Crystal X-ray Diffraction Facility established through a DAE-SRC Outstanding Investigator Award.References
- E. Lanzarotti, R. R. Biekofsky, D. A. Estrin, M. A. Marti and A. G. Turjanski, J. Chem. Inf. Model., 2011, 51, 1623 CrossRef CAS PubMed and references therein.
- C. A. Hunter, J. Singh and J. M. Thornton, J. Mol. Biol., 1991, 218, 837 CrossRef CAS; N. Kannan and S. Vishveshwara, Protein Eng., 2000, 13, 753 CrossRef PubMed; H. Krause, B. Ernstberger and H. J. Neusser, Chem. Phys. Lett., 1991, 184, 411 CrossRef; A. de Meijere and F. Huisken, J. Chem. Phys., 1990, 92, 5826 CrossRef PubMed; T. Iimori, Y. Aoki and Y. Ohshima, J. Chem. Phys., 2002, 117, 3675 CrossRef PubMed; O. Engkvist, P. Hobza, H. L. Selzle and E. W. Schlag, J. Chem. Phys., 1999, 110, 5758 CrossRef PubMed; C. Gonzalez and E. C. Lim, J. Phys. Chem. A, 2001, 105, 1904 CrossRef; T. P. Sauer and C. D. Sherrill, J. Phys. Chem. A, 2005, 109, 10475 CrossRef PubMed.
- S. Janich, R. Frohlich, A. Wakamiya, S. Yamaguchi and E. U. Wurthwein, Chem.–Eur. J., 2009, 15, 10457 CrossRef CAS PubMed.
- T. Morimoto, H. Uno and H. Furuta, Angew. Chem., Int. Ed. Engl., 2007, 46, 3672 CrossRef CAS PubMed; H. Uno, Y. Fumoto, K. Inoue and N. Ono, Tetrahedron, 2003, 59, 601 CrossRef; M. Sathiyendiran, J. Y. Wu, M. Velayudam, G. H. Lee, S. M. Peng and K. L. Lu, Chem. Commun., 2009, 3795 RSC.
- S. Kato, T. Nakagaki, T. Shimasaki and T. Shinmyozu, CrystEngComm, 2008, 10, 483 RSC; S. Kumar and A. Das, J. Chem. Phys., 2012, 136, 174302 CrossRef CAS PubMed.
- P. Elumalai, P. Rajakannu, F. Hussain and M. Sathiyendiran, RSC Adv., 2013, 3, 2171 RSC.
- C. Y. Su, Y. P. Cai, C. L. Chen, F. Lissner, B. S. Kang and W. Kaim, Angew. Chem., Int. Ed., 2002, 41, 3371 CrossRef CAS; D. Y. Lee, N. Singh, M. J. Kim and D. O. Jang, Org. Lett., 2011, 13, 3024 CrossRef PubMed; B. Shankar, F. Hussain and M. Sathiyendiran, J. Organomet. Chem., 2012, 719, 26 CrossRef PubMed; B. Shankar, P. Elumalai, R. Shanmugam and M. Sathiyendiran, J. Organomet. Chem., 2014, 749, 224 CrossRef PubMed; P. Rajakannu, P. Elumalai, B. Shankar, F. Hussain and M. Sathiyendiran, Dalton Trans., 2013, 42, 11359 RSC; P. Rajakannu, P. Elumalai, F. Hussain and M. Sathiyendiran, J. Organomet. Chem., 2013, 725, 1 CrossRef PubMed.
- X. Han, H. Ma and Y. Wang, Russ. J. Org. Chem., 2008, 44, 863 CrossRef CAS.
- J. Hua, H. Yao, Y. Bai, D. Zhao, S. Chena and J. Zhaoa, Polyhedron, 2014, 78, 1 CrossRef PubMed.
- C. L. Chen, J. Y. Zhang and C. Y. Su, Eur. J. Inorg. Chem., 2007, 2997 CrossRef CAS PubMed; M. Arunachalam, E. Suresh and P. Ghosh, Tetrahedron Lett., 2007, 48, 2909 CrossRef PubMed; M. Arunachalam and P. Ghosh, Chem. Commun., 2009, 3184 RSC; X. F. Wang, Y. Li, Z. Su, T. A. Okamura, G. Wu, W. Y. Sun and N. Ueyama, Z. Anorg. Allg. Chem., 2007, 633, 2695 CrossRef PubMed; G. C. Xu, Y. J. Ding, T. A. Okamura, Y. Q. Huang, G. X. Liu, W. Y. Sun and N. Ueyama, CrystEngComm, 2008, 10, 1052 RSC; W. Y. Sun, J. Fan, J. Hu, K. B. Yu and W. X. Tang, J. Chem. Crystallogr., 2000, 30, 115 CrossRef.
- A. D. Becke, Phys. Rev. A: At., Mol., Opt. Phys., 1988, 38, 3098 CrossRef CAS; A. D. Becke, J. Chem. Phys., 1993, 98, 5648 CrossRef PubMed; C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785 CrossRef; R. Ditchfield, W. J. Hehre and J. Pople, J. Chem. Phys., 1971, 54, 724 CrossRef PubMed; Y. Zhao and D. G. Truhlar, Theor. Chem. Acc., 2008, 120, 215 CrossRef.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision A.02, Gaussian, Inc., Wallingford, CT, 2009 Search PubMed.
- (a) L. J. Farrugia, WinGX, Version 1.80.05, J. Appl. Crystallogr. 1999, 32, 837 Search PubMed; (b) A. Altomare, G. Cascarano, C. Giacovazzo, A. Guagliardi, M. C. Burla, G. Polidori and M. Camalli, J. Appl. Crystallogr., 1994, 27, 435 Search PubMed; (c) G. M. Sheldrick, SHELXL-97, Program for crystal structure refinement, University of Göttingen, Göttingen, Germany, 1997 Search PubMed; (d) G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: X-ray crystallographic file in CIF format for 1, 3 and 5. Molecular structure of 1, optimized geometry, NMR spectra, and tables of XRD data. CCDC 1419799, 1051126 and 1051212. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra05151g |
| This journal is © The Royal Society of Chemistry 2015 |