Poly(vinylidene fluoride) nanocomposite capacitors with a significantly enhanced dielectric constant and energy density by filling with surface-fluorinated Ba0.6Sr0.4TiO3 nanofibers
Shaohui Liua,
Shi Xiaoa,
Shaomei Xiua,
Bo Shena,
Jiwei Zhai*a and
Zhenlian Anb
aKey Laboratory of Advanced Civil Engineering Materials of Ministry of Education, Functional Materials Research Laboratory, School of Materials Science & Engineering, Tongji University, 4800 Caoan Road, Shanghai 201804, China. E-mail: apzhai@tongji.edu.cn; Fax: +86 21 69584759; Tel: +86 21 69584759
bDepartment of Electrical Engineering, Tongji University, 4800 Caoan Road, Shanghai 201804, China
First published on 28th April 2015
Abstract
We report nanocomposites with an increased dielectric constant, enhanced electric breakdown strength and high-energy density based on a surface-modified Ba0.6Sr0.4TiO3 nanofiber (BST NF) filled poly(vinylidene fluoride) polymer. To improve the dispersion stability of the fillers in the polymer matrices, surface modification of the dielectric fillers was obtained through functionalization of the BST NF by hydroxylation using H2O2 treatment and subsequent fluorination. The surface-fluorinated BST NF (F-BST NF) exhibit excellent dispersion in the PVDF polymer matrix and strong interfacial adhesion with the matrix, leading to excellent flexibility for the composites. The composites exhibit the optimum dielectric performance (i.e. high dielectric constant and high breakdown strength). With 2.5 vol% of F-BST NF, the extractable energy density of the F-BST NF/PVDF composites is 7.5 J cm−3, which is three times as high as that of a pure PVDF matrix, showing good potential for energy storage applications. Our strategy provides a simple but effective way to prepare high performance flexible dielectric composite capacitors with high storage density.
Introduction
Flexible dielectric composite capacitors with high storage density play an important role in deriving the continuing development of advanced electronic devices and electric power systems.1–14 The energy density of dielectric material capacitors is defined as U = ∫EdD where E is the applied electric field and D is the electric displacement. On the basis of the equation, it is indicated that a large dielectric constant and breakdown strength are highly desired for high energy density.15,16 However, it is very difficult to optimize them synchronously. To date, polymer capacitors, such as biaxially oriented polypropylene (BOPP), are flexible and easy to process and have a high breakdown strength but are limited by their low dielectric constant, whereas ferroelectric ceramic capacitors, such as Pb(Zr,Ti)O3, Ba1−xSrxTiO3 and BaTiO3, have a high dielectric constant but are brittle and have a low breakdown strength.17,18To enhance the dielectric constants of the polymers, an effective way is introducing high dielectric constant ceramic fillers, such as Pb(Zr,Ti)O3, Ba1−xSrxTiO3 and BaTiO3, into the polymer matrix. However, to achieve high dielectric constants for nanocomposites, a relatively high volume fraction of ceramic filler (>50 vol%) is normally required, which inevitably raises the issues of inhomogeneity and aggregation in the fillers in the polymer matrix, resulting in many shortcomings such as poor mechanical properties, low breakdown strength. That leads to very minor enhancement of energy-storage density as compared with the pure polymer matrix. To overcome these disadvantages, a promising strategy has been developed by using the ceramic nanofibers with high aspect ratio rather than nanoparticles to enhance the dielectric energy storage density in polymer nanocomposites.19–21
The interface between the fillers and the polymer matrix is the key parameter in determining the energy storage density of composites.22 Achieving the ability to tailor the interface between organic polymer and inorganic fillers is the key issue to realize desirable dielectric properties and high energy density in the nanocomposites. PVDF based polymers are generally immiscible with most organic and inorganic materials because of the low surface energy of the fluoro-polymers. When nanofillers are added into the PVDF polymer, nanofillers tend to remain as entangled agglomerates, and homogeneous dispersion is not easily obtained due to the high surface energy of inorganic nanofillers. Furthermore, because of the great difference of intrinsic surface properties between the PVDF and inorganic filler, interfacial bonding between them is weak. Poor dispersion and weak interaction between ceramic filler and the surrounding matrix have negative influences on the dielectric properties and electric breakdown properties of the nanocomposites.
To solve the aforementioned problems, significant efforts have been devoted to prepare functionalized ceramic fillers.23–26 In this strategy, the polymers are grafted or attached onto the surfaces or edges of fillers, not only acting as the inter layers to prevent the agglomeration of the fillers, but also enhancing the compatibility between the fillers and the polymer matrix. Nevertheless, these methods also suffer from some drawbacks. For example, chemical modification and functionalization such as coupling agents on the ceramic nanofillers shows to be feasible and effective means of improving the solubility and dispersion of ceramic filler. However, the absorbed coupling agent on the filler surfaces are difficult to be removed completely, leading to high dielectric loss and high leakage current in the corresponding nanocomposites.27 Consequently, it is highly necessary to develop other facile and approaches to prepare polymer functionalized ceramic fillers for nanocomposite.
Surface-fluorination, as one of most effective approaches to surface modification of fillers, has been widely developed from fundamental researches, because of its extreme reactivity and oxidizing power. The research works show that surface-fluorination is useful to modify the interface areas between nanofillers and the polymer matrix.28,29 After surface-fluorination, the fluorine bonds will be formed on the MWCNT surface, which can act as the anchor to graft other functional molecules or polymers. Improved dispersion and integration of fluorinated nanotubes in composites has already been observed in the works of Zhu et al.28 and Meisha et al.,30 in epoxy and poly(ethylene oxide) matrixes, respectively. However, to the best of our knowledge, the utility of surface-fluorinated BST NF as modifier to enhance the dispersion of nanoparticles and improve the interfacial adhesion between the nanoparticles and PVDF matrix has not been reported. Moreover, the effect of the surface-fluorinated BST NF content on the composites of dielectric property and energy storage density of the polymer nanocomposites is not clear. In this work, we presented a simple strategy to fabricate flexible dielectric composite of high dielectric constant BST NF and matrix. The electrospun BST NF was sintered at about 900 °C to form perovskite crystalline. Surface modification of BST NF was obtained through functionalization of the BST NF by hydroxylation using H2O2 treatment and subsequent fluorination with F2/N2 mixed gas. Surface-fluorinated BST NF exhibit excellent dispersion in the PVDF polymer matrix and strong interfacial adhesion with the matrix, leading to excellent flexibility for the composites. The effects of content of modified BST NF on dielectric properties and energy-storage properties of the composites were investigated. In comparison with pure PVDF, the energy density increased more than double (from 2.8 to 7.5 J cm−3) when the loading of modified BST NF was 2.5 vol%, showing good potential for dielectric applications.
Experimental
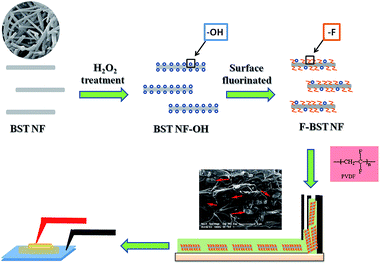
BST NF have been successfully synthesized by electrospinning.16,20 The BST NF were dispersed in an aqueous solution of H2O2 (35%, 350 mL) and heated to 100 °C for 3 h, and then centrifuged the solution. Then the hydroxylated BST NF (BST NF-OH) were reacted with F2 gas, forming surface fluorinated BST NF (F-BST NF). Surface fluorination of the BST NF was performed in a laboratory stainless vessel using a homemade mixture of F2/N2 gas (12.5% F2 by volume), at 0.1 MPa and temperature of 70 °C, for 60 min. For the fabrication of the BST NF/PVDF composites, the F-BST NF and PVDF powders (3F Co., China) were proportionally dispersed in N,N-dimethylformamide (DMF) under vigorous stirring at 40 °C for 10 h to make it stable and homogenous. The suspension was cast onto an indium tin oxide (ITO) glass and dried under vacuum at 60 °C for 10 h for solvent volatilizing. The obtained films were heated at 200 °C for 10 min then immediately quenched in ice water. The final quenched films were dried at 40 °C for 24 h. The nanocomposite films were about 10 μm in thickness. The schematic diagrams of the fabrication of F-BST NF/PVDF nanocomposites are shown in Fig. 1. The use of the surface fluorination effectively introduces passive interfaces between BST NF and PVDF matrix. Surface modification of dielectric fillers was obtained through functionalization of the BST NF by hydroxylation using H2O2 treatment and subsequent fluorination. Hydroxyl groups were introduced onto the surface of BST NF after treatment with H2O2. After surface fluorination, fluorine bonds were successfully grafted onto the hydroxyl group on the surface of BST NF. Fluorine groups attached to BST NF offer the opportunity for chemical interactions with the polymer systems. Robust bindings have been established between surface modified BST NF and PVDF matrix, which greatly improves the dispersion of the nanofibers.Characterization
X-ray diffraction (XRD) was applied to investigate the crystal structure of the samples with Cu-Kα radiation using a RIGAKU D/max2550 equipment. Microstructure of the samples was observed using field emission scanning electron microscopy (FESEM, XL30FEG, Philips, Netherlands). Thermogravimetric analysis (TGA) were conducted by a NETZSCH STA449C instrument under air atmosphere at the heating rate of 10 °C min−1. X-ray photoelectron spectroscopy (XPS) was used to verify the surface-fluorinated BST NF with Al Kα radiation (160 eV) using a Kratos Axis Ultra DLD multi-technique X-ray photoelectron spectrometer equipment. For electrical measurements, the top gold electrodes with 2 mm in diameter and a thickness of 40 nm were sputtered onto the surfaces of the nanocomposites films using a shadow mask. The bottom electrode was the ITO glass. Dielectric properties were measured by employing a E4980A LCR meter (Agilent, Palo Alto, CA, USA) in the frequency range of 100 Hz to 2 MHz with voltage amplitude of 500 mV at room temperature. P–E loops (polarization–electric field loops) were measured by a Premier II ferroelectric test system (Radiant Technologies, Inc.) in a silicone oil bath to avoid electrical discharges that would occur in air.Results and discussion
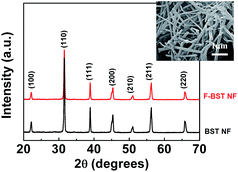
Fig. 2 presents the XRD patterns of the as-prepared BST NF synthesized by electrospinning and F-BST NF. No diffraction peaks from any other impurities were detected. Thus, the results clearly show that pure perovskite phase is obtained in the BST NF. XRD results exhibit no changes in the sample of the crystal structure of both F-BST NF and untreated BST NF. FESEM reveals that the nanofibers have the large aspect ratios, i.e., diameters of 100–150 nm and lengths of tens of micrometers, as seen from the FESEM image (inset of Fig. 2).TGA curves (Fig. 3) present the evidence to confirm the evolution of surface fluorination. It can be seen from Fig. 3 that (i) the weight loss of the nanofibers shows the sequence of BST NF < BST NF-OH < F-BST NF at 800 °C (weight loss of the samples was summarized in the inset), (ii) BST NF-OH show the maximum weight loss before 300 °C, indirectly confirming that the hydroxyl groups were introduced onto the surface of BST NF-OH.15 The fluorinated sample, F-BST NF, already indicates a two-step weight loss on the TGA plot (Fig. 3). The first step weight loss takes place before 300 °C and represents hydroxyl groups. The second weight loss step takes place at temperatures between 350 and 550 °C and represents the oxidation and vaporization of outer fluorine bond in the F-BST NF.29 In summary, comparison of the TGA data on BST NF-OH and F-BST NF shows that fluorine bond has been successfully introduced onto the surface of BST NF.
 | ||
| Fig. 3 TGA curves for the BST NF, BST NF-OH, and F-BST NF. Weight loss of the samples was summarized in the inset. | ||
Fig. 4a shows XPS survey spectra of the hydroxylated and fluorinated BST NF samples respectively. The BST NF-OH sample contains Sr, Ti, O, Ba and C elements, with sharp photoelectron peaks appearing at binding energies of 269 (Sr 3p), 458 (Ti 2p), 531 (O 1s), 780 (Ba 3d), and 285 eV (C 1s). The carbon peak is attributed to the residual carbon from the sample and adventitious hydrocarbon from the XPS instrument itself. On the contrary, the F-BST NF sample contains not only Sr, Ti, O, Ba and C, but also a small amount of F elements (binding energies at 684 eV), which come from the fluorination. The inset of Fig. 4a shows the high-resolution XPS spectrum of the F 1s region, taken on the surface of the fluorinated BST NF prepared. According to our previous results, the F 1s peak at 684 eV is due to the fluorine bond formed by reaction between F2 and the surface hydroxyl group on the surface of BST NF.31,32 The high-resolution XPS spectra of O 1s of BST NF-OH and F-BST NF are also plotted in Fig. 4b. One can see the peaks of O 1s (529.4 and 531.5 eV) corresponding to the O atoms in Ba0.6Sr0.4TiO3 (OBST) and free –OH(O–OH).24 The ratio of OBST/O–OH is about 1.10. After surface-fluorination, the ratio of OBST/O–OH is about 1.49, indicating that more than half of the free –OH groups reacted with the F2 gas. And the binding energy of the O 1s level is also shifted to a higher level depending on the fluorine concentration, suggesting that fluorine bond exist in the fluorinated BST NF.33
 | ||
| Fig. 4 (a) XPS spectra of BST NF-OH, and F-BST NF. High-resolution XPS of F 1s of F-BST NF is shown in the inset. (b) High-resolution XPS spectra of O 1s of BST NF-OH, and F-BST NF. | ||
The SEM images of the cross section of both nanocomposite with the F-BST NF and that with untreated BST NF at a concentration of 5 vol% suggest that the nanocomposite with the F-BST NF shows less agglomeration than that with untreated BST NF in Fig. 5. The F-BST NF are well distributed in the PVDF polymer matrix and show little agglomeration in Fig. 5a, meanwhile much more agglomeration is observed in the nanocomposite with untreated BST NF in Fig. 5b. This result indicates the F-BST NF could not only facilitate its dispersion in the polymer matrix but also strongly chain with the polymer matrix by fluorine bonds in the interface. The nanofibers have been successfully transferred to the polymer matrix with minimum agglomeration from solution and F-BST NF tend to orient in the in-plane directions of the composite films.
 | ||
| Fig. 5 SEM images of the cross section of the nanocomposite film with the F-BST NF (a) and that with untreated BST NF (b) at a concentration of 5 vol%. | ||
The dielectric constant and loss tangent of the composites at 1 kHz as a function of content with untreated BST NF and F-BST NF are shown in Fig. 6. As seen, the dielectric constant of the pure PVDF is low (7.9 at 1 kHz). The dielectric constant of the composites gradually increases with the content of F-BST NF within a wide range from 2.5 vol% to 7.5 vol%, which means it can be tuned by changing the F-BST NF content. The maximum dielectric constant is 22.5 at small loading (7.5 vol%) F-BST NF, which is 2.84 times higher than that of the pure PVDF. This remarkable enhancement should be attributed to the considerably higher dielectric constant of the F-BST NF in comparison with the polymer matrix and the other high aspect ratio of ceramic filler.34 The dielectric constant of a composite is well described by effective medium theory, where the depolarization factor is strongly dependent on the aspect ratio of the fillers in the composite.4,21 Compared with the BST NF/PVDF, the F-BST NF/PVDF nanocomposite film with the same filler loading content not only shows a higher dielectric constant but also has a lower dielectric loss at a frequency of 1 kHz. The result should be attributed to the use of surface fluorination. Previous TG and XPS results indicate that fluorinated bonds were introduced onto the surface of BST NF. The surface fluorination benefits the homogenous distribution of BST NF in the polymer matrix. Compared to the composite film with the F-BST NF, the BST NF/PVDF have considerably more agglomeration and defects such as voids in the composite film, which is more responsible for lower dielectric constants and higher dielectric losses.16,35
 | ||
| Fig. 6 Dielectric constants (a) and dielectric loss (b) of BST NF/PVDF and F-BST NF/PVDF nanocomposite films loaded with various concentrations of fillers measured at 1 kHz. | ||
Theoretically, the polarization of linear dielectrics is linearly dependent on the applied electric field. The energy storage density of the linear dielectrics can be simply expressed as 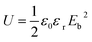 , where εr is the dielectric constant and Eb is the breakdown strength. However, linear dielectrics do not have high dielectric constant, which makes it difficult to obtain high energy density. For ferroelectric materials, the polarization and dielectric constant have strong dependence on the applied electric field. Therefore, the energy storage density should be calculated from the P–E loops. The energy densities of the nanocomposites can be computed from the D–E loops based on the following formula U = ∫EdD, where U is the discharged energy density.
, where εr is the dielectric constant and Eb is the breakdown strength. However, linear dielectrics do not have high dielectric constant, which makes it difficult to obtain high energy density. For ferroelectric materials, the polarization and dielectric constant have strong dependence on the applied electric field. Therefore, the energy storage density should be calculated from the P–E loops. The energy densities of the nanocomposites can be computed from the D–E loops based on the following formula U = ∫EdD, where U is the discharged energy density.
The breakdown strength is the key parameter in determining the energy storage density of nanocomposites. As a result of the improved breakdown strength, the nanocomposites could be polarized under higher electric fields, giving rise to larger polarization. Fig. 7 shows the breakdown strength for composites with different amounts of untreated BST NF and F-BST NF. All the composites of F-BST NF maintain a relatively high breakdown strength. The breakdown strength for the composite of 2.5 vol% F-BST NF reaches 3900 kV cm−1. Largely, this is because the F-BST NF with large aspect ratio tend to orient in the in-plane directions of the composite films during the solution cast process. When the electric field is applied in the out-of plane direction of the composite films, the susceptibility of the composite films could be reduced by the F-BST NF perpendicular to the external electric field, leading to a lower concentration of electric field in the polymer matrix. However, as the concentration of the filler increases, the breakdown strength of the nanocomposite films decreases sharply. Defects such as microcracks introduced with the fillers could lead to the concentration of the local electric charge reducing the breakdown strength, and a high loading of fillers raises the chance of interconnection between the fibers to facilitate charge transfer along the electric field direction. It should also be noted that the breakdown strength of the composites with the F-BST NF is higher than that with untreated BST NF. The improvement of the breakdown strength should be attributed to the surface modification. The surface fluorination could facilitate the dispersion of the BST NF in the polymer matrix and strongly chain with the polymer matrix by chemical bonds in the interface, which reduces the percolation pathways for the charge transfer and the mobility of the polymer chains.
 | ||
| Fig. 7 Breakdown strength for BST NF/PVDF and F-BST NF/PVDF nanocomposite films loaded with various concentrations of fillers. | ||
Fig. 8 illustrates the P–E loops of the composite films with different various volume fractions of F-BST NF measured at 100 Hz under the highest available electric field, where for each P–E curve the lower branch corresponds to the charging cycle and the upper branch corresponds to the discharging cycle. The polarization and remnant polarization of F-BST NF/PVDF nanocomposite films loaded with various concentrations fillers are shown in the inset of Fig. 8. Under the electric field of 2900 kV cm−1, the electric displacement of the nanocomposite films consistently increases with the volume fraction of F-BST NF. It is attributed to the fact that the dielectric constant of BST NF is larger than that of PVDF. It can also be observed that the remnant polarizations of the P–E loops increase with the volume fraction of the F-BST NF. The high remnant polarization will decrease the discharging energy and discharge efficiency of the material since the integrated area of the P–E loops will decrease. This is one of the reasons that the nanocomposite films used here have been synthesized with low volume fractions of F-BST NF.
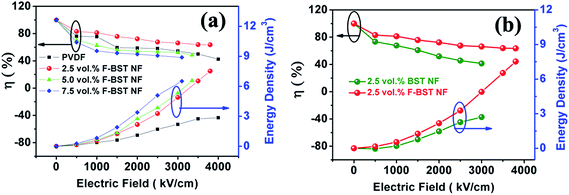
The energy densities of nanocomposite films calculated from the P–E loops as a function of applied field at different volume fractions is shown in Fig. 9a. Under the electric field of 2900 kV cm−1, the energy densities of the nanocomposite films increase with increasing electric field and volume fraction of the F-BST NF. Fig. 9a clearly shows that F-BST NF can significantly improve the energy density of the nanocomposite compared to a neat polymer. The maximal energy storage density of 7.5 J cm−3 was obtained in the nanocomposites with 2.5 vol% F-BST NF at 3900 kV cm−1, which is three times as high as that of pure PVDF matrix (2.8 J cm−3 at 4000 kV cm−1). This value is more than six times as large as high performance commercial polypropylene capacitors (1.2 J cm−3 at 6400 kV cm−1).8
For practical applications of dielectric capacitors in practice, the high field efficiency (η) is another important factor to characterize the energy storage performances of dielectric materials, since the energy losses in the capacitor leads to heating and, consequently, to detrimental effects on the performance and reliability of the capacitor. Fig. 9a gives the energy storage density and efficiency (discharge energy/charge energy) of the nanocomposite films with different F-BST NF concentrations with increasing of the electric field. It is clearly shown that the efficiency decreases with the applied electric filed, which is highly related to the conduction loss. As the concentration of the filler increases, the efficiency of the nanocomposite films decreases due to the larger hysteresis in the polarization. However, the efficiency of the PVDF film exhibits considerable reduction from 75% at 1000 kV cm−1 to 42% at 4000 kV cm−1, which indicates the rapid increase of energy loss at high fields due to conduction loss. Although the nanocomposite films with 2.5 vol% F-BST NF shows no apparent decrease with the electric filed. It is stable above 82% at electric fields below 1000 kV cm−1 and can still be maintained at 60% even at 3900 kV cm−1. Therefore, unlike a pure PVDF film, the nanocomposites show a marked reduction of loss at high fields while maintaining a high energy storage density.
Fig. 9b gives the discharged energy density and energy efficiency for nanocomposite films with the F-BST NF and those with untreated BST NF under various applied electric fields. It can be seen that, compared with nanocomposites with the untreated BST NF, F-BST NF/PVDF nanocomposites exhibit much higher energy efficiencies. More importantly, the 2.5 vol% F-BST NF/PVDF nanocomposites show much higher discharged energy densities at high electric field in comparison with those with untreated BST NF. This can be understood from the higher discharged energy densities of the F-BST NF/PVDF nanocomposite that arises from improved filler/matrix interfacial adhesion and dispersion of the filler and reduced conduction loss. While the hysteresis loss is related to conduction loss of the nanocomposites. These results clearly show the high effectiveness of surface fluorination on the enhancement of energy density of PVDF nanocomposites.
Conclusions
Achieving the ability to tailor the interface between organic polymer and inorganic fillers is the key issue to realize desirable dielectric properties and high energy storage density in the nanocomposites. A novel strategy to improve the interface effect of BST NF/polyvinylidene fluoride (PVDF) nanocomposites is developed to achieve high energy density. Surface modification of dielectric fillers was obtained through functionalization of the BST NF by hydroxylation using H2O2 treatment and subsequent fluorination. Surface fluorination could facilitate the homogeneous dispersion of the nanofiber fillers in the PVDF polymer matrix. The enhanced dielectric constant, reduced loss tangents and improved breakdown strength were obtained in PVDF-based nanocomposites filled with F-BST NF. Moreover, the energy storage density of 7.5 J cm−3 was obtained in the composite film with 2.5 wt% F-BST NF, which is three times as high as that of pure PVDF matrix. Such significant enhancement results from the combined effect of the large aspect ratio, the F-BST NF and the homogeneous dispersion of the nanofiber fillers in the PVDF polymer matrix. The results suggest that the BST NF with the high aspect ratio, by employing surface fluorination, can be used to improve the energy storage density of nanocomposites, thus providing a route for using the ceramic nanofibers to enhance the dielectric energy storage density in polymer nanocomposites.Acknowledgements
This research was supported by the Ministry of Sciences and Technology of China through 973-project under Grant (2015CB654601).References
- B. J. Chu, X. Zhou, K. L. Ren, B. Neese, M. R. Lin, Q. Wang, F. Bauer and Q. M. Zhang, A dielectric polymer with high electric energy density and fast discharge speed, Science, 2006, 313, 334–336 CrossRef CAS PubMed.
- Z. M. Dang, J. K. Yuan, J. W. Zha, T. Zhou, S. T. Li and G. H. Hu, Fundamentals, processes and applications of high-permittivity polymer matrix composites, Prog. Mater. Sci., 2012, 57, 660–723 CrossRef CAS PubMed.
- X. Y. Huang and P. K. Jiang, Core–Shell Structured High-k Polymer Nanocomposites for Energy Storage and Dielectric Applications, Adv. Mater., 2014 DOI:10.1002/adma.201401310.
- B. C. Luo, X. H. Wang, Y. P. Wang and L. T. Li, Fabrication, characterization, properties and theoretical analysis of ceramic/PVDF composite flexible films with high dielectric constant and low dielectric loss, J. Mater. Chem. A, 2014, 2, 510–519 CAS.
- H. Y. Liu, Y. Shen, Y. Song, C. W. Nan, Y. H. Lin and X. P. Yang, Carbon Nanotube Array/Polymer Core/Shell Structured Composites with High Dielectric Permittivity, Low Dielectric Loss, and Large Energy Density, Adv. Mater., 2011, 23, 5104–5108 CrossRef CAS PubMed.
- P. H. Hu, Y. Song, H. Y. Liu, Y. Shen, Y. H. Lin and C. W. Nan, Largely enhanced energy density in flexible P(VDF-TrFE) nanocomposites by surface-modified electrospun BaSrTiO3 fibers, J. Mater. Chem. A, 2013, 1, 1688–1693 CAS.
- H. X. Tang, Y. R. Lin and H. A. Sodano, Enhanced Energy Storage in Nanocomposite Capacitors through Aligned PZT Nanowires by Uniaxial Strain Assembly, Adv. Energy Mater., 2012, 2, 469–476 CrossRef CAS PubMed.
- H. X. Tang and H. A. Sodano, Ultra High Energy Density Nanocomposite Capacitors with Fast Discharge Using Ba0.2Sr0.8TiO3 Nanowires, Nano Lett., 2013, 13, 1373–1379 CAS.
- P. Kim, S. C. Jones, P. J. Hotchkiss, J. N. Haddock, B. Kippelen, S. R. Marder and J. W. Perry, Phosphonic acid-modified barium titanate polymer nanocomposites with high permittivity and dielectric strength, Adv. Mater., 2007, 19, 1001–1005 CrossRef CAS PubMed.
- K. Yu, Y. J. Niu, Y. Y. Bai, Y. C. Zhou and H. Wang, Poly(vinylidene fluoride) polymer based nanocomposites with significantly reduced energy loss by filling with core–shell structured BaTiO3/SiO2 nanoparticles, Appl. Phys. Lett., 2013, 102, 102903–102905 CrossRef PubMed.
- F. Wen, Z. Xu, S. B. Tan, W. M. Xia, X. Y. Wei and Z. C. Zhang, Chemical Bonding-Induced Low Dielectric Loss and Low Conductivity in High-K Poly(vinylidenefluoride-trifluorethylene)/Graphene Nanosheets Nanocomposites, ACS Appl. Mater. Interfaces, 2013, 5, 9411–9420 CAS.
- V. K. Thakur, J. Yan, M. F. Lin, C. Y. Zhi, D. Golberg, Y. Bando, R. Sim and P. S. Lee, Novel polymer nanocomposites from bioinspired green aqueous functionalization of BNNTs, Polym. Chem., 2012, 3, 962–969 RSC.
- V. K. Thakur, G. Q. Ding, J. Ma, P. S. Lee and X. H. Lu, Hybrid Materials and Polymer Electrolytes for Electrochromic Device Applications, Adv. Mater., 2012, 24, 4071–4096 CrossRef CAS PubMed.
- M. F. Lin, V. K. Thakur, E. J. Tan and P. S. Lee, Dopant induced hollow BaTiO3 nanostructures for application in high performance capacitors, J. Mater. Chem., 2011, 21, 16500–16504 RSC.
- M. N. Almadhoun, U. S. Bhansali and H. N. Alshareef, Nanocomposites of ferroelectric polymers with surface-hydroxylated BaTiO3 nanoparticles for energy storage applications, J. Mater. Chem., 2012, 22, 11196–11200 RSC.
- S. H. Liu, J. W. Zhai, J. W. Wang, S. X. Xue and W. Q. Zhang, Enhanced Energy Storage Density in Poly(Vinylidene Fluoride) Nanocomposites by a Small Loading of Surface-Hydroxylated Ba0.6Sr0.4TiO3 Nanofibers, ACS Appl. Mater. Interfaces, 2014, 6, 1533–1540 CAS.
- D. C. Zhang, X. Zhang, Y. Chen, P. Yu, C. H. Wang and Y. W. Ma, Enhanced capacitance and rate capability of graphene/polypyrrole composite as electrode material for supercapacitors, J. Power Sources, 2011, 196, 5990–5996 CrossRef CAS PubMed.
- D. C. Zhang, X. Zhang, Y. Chen, P. Yu, C. H. Wang and Y. W. Ma, Enhanced capacitance and rate capability of graphene/polypyrrole composite as electrode material for supercapacitors, J. Power Sources, 2013, 239, 561 CrossRef CAS PubMed.
- S. H. Liu, S. X. Xue, W. Q. Zhang and J. W. Zhai, Enhanced dielectric and energy storage density induced by surface-modified BaTiO3 nanofibers in poly(vinylidene fluoride) nanocomposites, Ceram. Int., 2014, 40, 15633–15640 CrossRef CAS PubMed.
- S. H. Liu and J. W. Zhai, A small loading of surface-modified Ba0.6Sr0.4TiO3 nanofiber-filled nanocomposites with enhanced dielectric constant and energy density, RSC Adv., 2014, 4, 40973–40979 RSC.
- Y. Song, Y. Shen, H. Y. Liu, Y. H. Lin, M. Li and C. W. Nan, Improving the dielectric constants and breakdown strength of polymer composites: effects of the shape of the BaTiO3 nanoinclusions, surface modification and polymer matrix, J. Mater. Chem., 2012, 22, 16491–16498 RSC.
- T. Zhou, J. W. Zha, R. Y. Cui, B. H. Fan, J. K. Yuan and Z. M. Dang, Improving Dielectric Properties of BaTiO3/Ferroelectric Polymer Composites by Employing Surface Hydroxylated BaTiO3 Nanoparticles, ACS Appl. Mater. Interfaces, 2011, 3, 2184–2188 CAS.
- Z. M. Dang, Y. J. Xia, J. W. Zha, J. K. Yuan and J. B. Bai, Preparation and dielectric properties of surface modified TiO2/silicone rubber nanocomposites, Mater. Lett., 2011, 65, 3430–3432 CrossRef CAS PubMed.
- L. Y. Xie, X. Y. Huang, Y. H. Huang, K. Yang and P. K. Jiang, Core–shell Structured Hyperbranched Aromatic Polyamide/BaTiO3 Hybrid Filler for Poly(vinylidene fluoride-trifluoroethylene-chlorofluoroethylene) Nanocomposites with the Dielectric Constant Comparable to That of Percolative Composites, ACS Appl. Mater. Interfaces, 2013, 5, 1747–1756 CAS.
- P. Kim, N. M. Doss, J. P. Tillotson, P. J. Hotchkiss, M. J. Pan, S. R. Marder, J. Y. Li, J. P. Calame and J. W. Perry, High Energy Density Nanocomposites Based on Surface-Modified BaTiO3 and a Ferroelectric Polymer, ACS Nano, 2009, 3, 2581–2592 CrossRef CAS PubMed.
- S. Liu and J. Zhai, Improving the dielectric constant and energy density of poly(vinylidene fluoride) composites induced by surface-modified SrTiO3 nanofibers by polyvinylpyrrolidone, J. Mater. Chem. A, 2015, 3, 1511–1517 CAS.
- Z. M. Dang, H. Y. Wang and H. P. Xu, Influence of silane coupling agent on morphology and dielectric property in BaTiO3/polyvinylidene fluoride composites, Appl. Phys. Lett., 2006, 89, 112902–112903 CrossRef PubMed.
- J. Zhu, J. D. Kim, H. Q. Peng, J. L. Margrave, V. N. Khabashesku and E. V. Barrera, Improving the dispersion and integration of single-walled carbon nanotubes in epoxy composites through functionalization, Nano Lett., 2003, 3, 1107–1113 CrossRef CAS.
- Y. Liu, R. L. Vander Wal and V. N. Khabashesku, Functionalization of carbon nano-onions by direct fluorination, Chem. Mater., 2007, 19, 778–786 CrossRef CAS.
- M. L. Shofner, V. N. Khabashesku and E. V. Barrera, Processing and mechanical properties of fluorinated single-wall carbon nanotube–polyethylene composites, Chem. Mater., 2006, 18, 906–913 CrossRef CAS.
- J. G. Yu, Q. J. Xiang, J. R. Ran and S. Mann, One-step hydrothermal fabrication and photocatalytic activity of surface-fluorinated TiO2 hollow microspheres and tabular anatase single micro-crystals with high-energy facets, CrystEngComm, 2010, 12, 872–879 RSC.
- J. G. Yu, W. G. Wang, B. Cheng and B. L. Su, Enhancement of Photocatalytic Activity of Mesoporous TiO2 Powders by Hydrothermal Surface Fluorination Treatment, J. Phys. Chem. C, 2009, 113, 6743–6750 CAS.
- D. S. Kim, J. Lee and K. Char, Electrical characteristics of SiOxFy gate oxides formed by a plasma fluorination technique, Appl. Phys. Lett., 2005, 87, 042107 CrossRef PubMed.
- S. H. Liu, S. X. Xue, W. Q. Zhang, J. W. Zhai and G. H. Chen, Significantly enhanced dielectric property in PVDF nanocomposites flexible films through a small loading of surface-hydroxylated Ba0.6Sr0.4TiO3 nanotubes, J. Mater. Chem. A, 2014, 2, 18040–18046 CAS.
- K. Yu, Y. J. Niu, Y. C. Zhou, Y. Y. Bai and H. Wang, Nanocomposites of Surface-Modified BaTiO3 Nanoparticles Filled Ferroelectric Polymer with Enhanced Energy Density, J. Am. Ceram. Soc., 2013, 96, 2519–2524 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |