Three-dimensionally ordered macroporous (3DOM) SiOC on a cordierite monolith inner wall and its properties for soot combustion†
Jia Maa,
Yunlong Ninga,
Cairong Gong*a,
Gang Xueb and
Guoliang Fana
aSchool of Material Science and Engineering, Tianjin University, Tianjin 300072, P. R. China. E-mail: gcr@tju.edu.cn; Fax: +86 22 27404724; Tel: +86 22 27404226
bInstitute of Power Source & Ecomaterials Science, Hebei University of Technology, Tianjin 300130, P. R. China
First published on 2nd June 2015
Abstract
Three-dimensionally ordered macroporous (3DOM) SiOC was successfully fabricated on a cordierite monolith inner wall of a polystyrene (PS) sphere template through a colloidal crystal templating (CCT) method (named as 3DOM SiOC/cordierite). And perovskite-type LaCoO3 (LCO) mixed metal oxides, prepared by a facile sol–gel process, were coated onto the wall of the 3DOM SiOC/cordierite by a simple impregnation-sintering approach (named as LCO/3DOM SiOC/cordierite). The morphological and physicochemical properties studies indicate that the 3DOM structure has excellent connectivity, a large specific surface area, and applicable pore size. And the 3DOM structure plays an important role in preventing aggregation of the targeted catalyst particles, improving the contact condition between catalysts and soot as well as enhancing the reducibility of the LCO. The subsequent investigation on the catalytic performance of the 3DOM samples for diesel soot combustion shows that the 3DOM texture offers the catalyst a lower soot combustion temperature than a conventional catalyst, and the LCO/3DOM SiOC/cordierite reduces the combustion temperature even further while avoiding the rise of back-pressure effectively as well.
1. Introduction
Diesel engines have dominated heavy-duty trucks and off-road vehicles all over the world due to their great advantages which surpass other engine types, including longer durability, lower costs, and higher efficiency. Diesel particulate matter (PM, mainly containing soot) and nitrogen oxides (NOx), undesired by-products of diesel engines from the combustion process, have been recognized among the top threats to the environment and human health which has driven research focusing on solutions.1,2A diesel particulate filter (DPF) is an effective technology in engine development to reduce diesel soot on-site efficiently. As a physical entrapment, the on-site capture of soot in a DPF requires a desired durability or continuous regeneration, as the increase of back-pressure from a blocking trap will damage the engine.3 However, passive regeneration generally starts at a temperature above 550 °C which is much higher than the exhaust-gas temperature (200–400 °C) under the normal working conditions of the engine. Catalyst coatings were commonly used to lower the regeneration temperature, which enable continuous regeneration under normal working conditions.4–7 Some catalysts exhibited excellent performance in the simultaneous removal of soot and NOx.8–10 Several factors of catalysts preliminarily govern the catalytic activity: chemical composition, specific surface area,11 grain size,12 surface contact condition with soot,13,14 as well as porosity and pore structure.15 Additionally, being a soot–catalyst–gas three-phase reaction, the fluidity and permeability of the gas are also important factors in the soot combustion process. Over decades of fundamental study and practical application demonstrate that catalytic coatings over DPFs are an efficient after-treatment technique. Unfortunately, normal coating techniques often provide a catalyst coating with a small specific surface area, small pore size and an unsatisfactory contact condition with soot and thus limit their catalytic activity. Furthermore, catalyst particles agglomerate easily and block the DPF wall channels, resulting in the deterioration of the fluidity of the gas and the increase of back-pressure which eventually overheat the engine.
Three-dimensionally ordered macroporous (3DOM) materials have demonstrated excellent properties, such as an open connected macropore structure and nanosized wall components,16 and numerous successful applications have benefited from these properties. As for the features of 3DOM materials, 3DOM catalysts showed better properties than conventional catalysts, as a result of higher specific area, better surface contact condition with soot, excellent connectivity and a pore structure.17–21 3DOM structure offers an efficient way to improve the properties of the catalysts. To date, various kinds of 3DOM catalysts have been successfully fabricated by different methods. M. Sadakane et al.19 prepared 3DOM perovskite-type La1−xSrxFeO3 (x = 0–0.4) mixed metal oxides by using a new facile colloidal crystal templating method, and compared with nonporous catalysts, the T50 (a temperature where half of the carbon was burned) of the catalyst was reduced by 16 °C and the specific surface area was increased by 30 m2 g−1, demonstrating that the 3DOM perovskite-type materials have superior catalytic activity for the combustion of nanosized carbon. Y. Wei et al.20 successfully fabricated a series of catalysts of 3DOM Ce0.8Zr0.2O2-supported gold nanoparticles with controllable sizes by a gas bubbling-assisted membrane reduction (GBMR); as reported, 3DOM Au0.04/Ce0.8Zr0.2O2 may achieve the lowest T10 (a temperature where 10% of the carbon was burned, 218 °C) for soot oxidation under the loose contact condition.
Motivated by the analysis mentioned above, in this study, the 3DOM structure was applied in the catalyst coatings process for a real-life DPF device. A 3DOM SiOC structure was firstly fabricated on the inner wall of the cordierite monolith through a colloidal crystal templating method, following another coating of the LCO to reduce the combustion temperature of soot. The 3DOM structure showed excellent connectivity, a large specific surface area, and applicable pore size. The 3DOM structure also effectively restrained the aggregation of the catalyst particles, leading to the improved surface contact condition between catalysts and soot as well as the reducibility of LCO. Moreover, as the 3DOM structure formed on the cordierite walls, LCO/3DOM SiOC/cordierite effectively prohibits the chance for back-pressure to increase and further enhances the catalytic activity.
2. Experimental section
2.1. Synthesis of 3DOM SiOC cordierite
The general scheme of a synthesis of 3DOM SiOC/cordierite is relatively straightforward: dip-coating a SiOC thin film onto the cordierite monolith inner wall, depositing a closed-packed polystyrene (PS) spheres template onto the SiOC coating, filling the empty volume between the PS spheres with SiOC solution and then removing the PS spheres. All reagents used below are of analytic grade.For preparation of the PS spheres, tetrabutyl titanate was added into a water/ethanol (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 in volume) solution, whose pH was adjusted to 9 with NaOH solution (1 mol L−1), then it was stirred at 40 °C for 40 min. After that, styrene (purified by vacuum distillation), sodium dodecyl sulfate and ammonium persulfate (provided by Tianjin Guangfu Fin Chemicals Research Institute) were added, followed by stirring at 80 °C for 8 h under a nitrogen atmosphere.
3 in volume) solution, whose pH was adjusted to 9 with NaOH solution (1 mol L−1), then it was stirred at 40 °C for 40 min. After that, styrene (purified by vacuum distillation), sodium dodecyl sulfate and ammonium persulfate (provided by Tianjin Guangfu Fin Chemicals Research Institute) were added, followed by stirring at 80 °C for 8 h under a nitrogen atmosphere.
The second step was to coat SiOC onto the cordierite monoliths. The SiOC precursor solution was prepared by simply mixing polymethylhydrosiloxane (PMHS), 1,3,5,7-tetravinyl-1,3,5,7-tetramethylcyclotetrasiloxane (D4Vi) and a Karstedt catalyst solution (provided by Aladdin), which were then ultrasonically dispersed. The cordierite monoliths (provided by Yixing Jintai Refractory Material Co., Ltd), having 64 cells per square centimeter, were cut into pieces of 1 cm2 in section and 1.5 cm long. The pieces were first washed in an acetone/ethanol (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in volume) solution for 3 h and then in nitric acid for another 3 h. Finally, after being washed with deionized water, the cordierite monolith pieces were dried at 60 °C for 4 h. Then the pretreated monoliths were immersed into the prepared SiOC precursor solution for 30 min to coat the SiOC thin film, followed by being blown to remove the excess of slurry. After that, the monoliths were dried at 60 °C for 4 h. The cycle of immersion–blowing–drying was repeated three times. We named them SiOC/cordierite monoliths.
1 in volume) solution for 3 h and then in nitric acid for another 3 h. Finally, after being washed with deionized water, the cordierite monolith pieces were dried at 60 °C for 4 h. Then the pretreated monoliths were immersed into the prepared SiOC precursor solution for 30 min to coat the SiOC thin film, followed by being blown to remove the excess of slurry. After that, the monoliths were dried at 60 °C for 4 h. The cycle of immersion–blowing–drying was repeated three times. We named them SiOC/cordierite monoliths.
The prepared SiOC/cordierite monoliths were immersed into 100 mL PS precursor solution (35 wt%) in the centrifugation tubes, followed by centrifuging at 4000 rpm for 24 h to coat a thin film of PS spheres. After centrifugation, the monoliths were blown at 60 °C for 72 h to remove the excess of slurry. Then, the coated monoliths were soaked in the SiOC precursor solution for 5 days. After blowing away excessive solution, the obtained samples were incubated at 50 °C for 4 h. The product was crosslinked SiOC/cordierite monoliths and PS spheres.
To obtain 3DOM SiOC/cordierite monoliths, the crosslinked monoliths were heat-treated at 800 °C for 1 h under a nitrogen atmosphere and then calcined at 800 °C for 2 h in air.
2.2. Synthesis of the perovskite-type LaCoO3 (LCO) mixed metal oxides supported on 3DOM SiOC/cordierite
The perovskite-type oxides of LaCoO3 (LCO) were prepared by the sol–gel method.22 The 3DOM SiOC/cordierite monoliths were immersed into the catalyst precursor solution prepared previously for 30 min, and then were preheated at 100 °C for 1 h; after preheating, the samples were sintered at 450 °C for 1 h. The immersion–preheating–sintering–cooling cycle was repeated for several times to obtain a maximum upload. After uploading, the samples were calcined at 800 °C for 2 h. The products were named LCO/3DOM SiOC/cordierite.2.3. Structure characterization
The microstructures of the catalysts and 3DOM samples were observed by scanning electron microscopy (SEM, HitachiS4800, HITACHI, Japan) using accelerating voltages of 0.5–30 kV, and 0.1 kV per step, the samples (ground into powder) were glued to the sample holder by a conducting resin and then coated with a gold layer to improve the images obtained. The elemental chemical analysis was performed using the energy-dispersive X-ray analysis (EDX, Genesis XM2, EDAX, USA) system, which was equipped with the SEM instrument. The porosity, pore volume and BET surface areas were measured by N2 adsorption at −196 °C using a NOVA 2000 gas sorption analyzer, and the pore-size distribution (PSD) of samples was calculated using the Barrett–Joyner–Halenda (BJH) algorithm. Specific surface areas were calculated using the Brunauer–Emmett–Teller (BET) method. The crystal phases of the prepared catalysts were identified on an X-ray diffractometer (XRD D/MAX-2500, Rigaku, Tokyo, Japan) using Cu-Kα radiation. The XRD patterns were recorded for 2θ angles between 10° and 90° with increments of 0.02° and a counting time of 0.5 s−1 per step. The structures of the prepared catalysts were analyzed by Fourier transform infrared spectrometry (FTIR, Nicolet 6700, USA) with 2 cm−1 resolution. The IR wafers were prepared by mixing KBr powder with LCO (ca. 1% sample in KBr).Hydrogen temperature-programmed reduction (H2-TPR) experiments were carried out on a ChemBET Pulsar TPD/TPR analyzer (Quantachrome). 20 mg of LCO or 200 mg of LCO/3DOM SiOC (40–60 mesh) was loaded into a fixed-bed-U-shaped quartz microreactor. The sample was pretreated in a helium flow of 30 mL min−1 at 500 °C for 30 min and cooled under the same atmosphere to room temperature. The pretreated sample was exposed to a flow (50 mL min−1) of 5% H2 balanced Ar and heated from 50 °C to 750 °C at a rate of 10 °C min−1. The variation in H2 concentration of the effluent was monitored on-line by the chemical adsorption analyzer.
2.4. The catalytic activity test
The activity of the 3DOM samples and catalysts for carbon particle combustion were tested using a TG-DSC (Mettler Toledo, USA). 20 mg of the prepared samples were placed in an alumina crucible, then were heated under a gas flow (100 mL min−1) containing 10% O2 and 500 ppm NO balanced N2 at a rate of 10 °C min−1 at a temperature range of 50 °C to 800 °C. The performance of the samples was evaluated using the values of Tm, T10, T50, and T90; Tm was defined as the temperature at which the conversion rate of carbon black was the most rapid, and T10, T50 and T90 were defined as the temperatures at which 10%, 50% and 90% of carbon black was oxidized, respectively. Carbon black, purchased from Evonik Degussa, was used in the test as a replacement of diesel soot due to its similar chemical components and specific surface area. Before being used, carbon black was pretreated at 100 °C for 3 h. To imitate the real situation, carbon black was dispersed into anhydrous ethanol, and 3DOM contact was obtained by immersing the 3DOM samples into the suspension solution, and doing ultrasonic dispersion for 30 min to make the carbon black disperse onto the inner walls of the 3DOM samples. As a comparison, the catalytic activity of catalyst powders (catalyst to carbon black 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, mass ratio, loose contact) was also studied.
1, mass ratio, loose contact) was also studied.
3. Results and discussion
3.1. The microstructure of the 3DOM samples
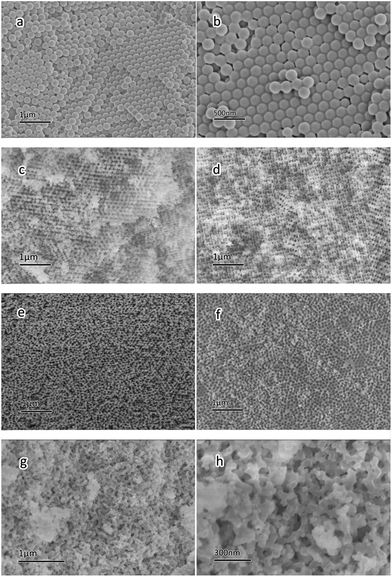
The morphology and pore structure of the PS microspheres and 3DOM samples (3DOM SiOC, 3DOM SiOC/cordierite and LCO/3DOM SiOC/cordierite) were investigated by SEM.Fig. 1a and b show the SEM images of PS microspheres, they depict a dispersion of monodisperse PS microspheres assembled into close-packed arrays. It shows a face-centered cubic (fcc) structure.23 The diameter of the PS spheres measured from the SEM images is in the range of 120 to 150 nm.
Fig. 1c–f all demonstrate that the samples obtained by the CCT method have the 3DOM structure. Concretely, Fig. 1c gives the SEM image of 3DOM SiOC sintered at 800 °C in Ar atmosphere. It can be seen that the 3DOM SiOC exactly copies the fcc structure of the PS template. As for the ideal model of the fcc structure, this structure highly improves the specific surface area and negotiability of gas flow.
It can also be found that the pore size, wall thickness and morphology varied under different treatments, as listed in Table 1. From Table 1 and Fig. 1c, it can be seen that the wall thickness ranges from 50 to 100 nm, indicating a sheet structure. Macropore diameters measured from the SEM images range from 100 to 150 nm, which are narrow compared with the size of the PS microspheres, and the macropores show irregular roundness. It might be caused by melting of the PS microspheres during sintering.21
| Sample | Sphere (pore) size/nm | Wall thickness/nm |
|---|---|---|
| PS microspheres | 120–150 | — |
| 3DOM SiOC (before annealing) | 100–150 | 50–100 |
| 3DOM SiOC (after annealing) | 100–400 | 20–100 |
| 3DOM SiOC/cordierite | 100–120 | 40–50 |
| LCO/3DOM SiOC/cordierite | 200–600 | — |
Fig. 1d shows the SEM image of 3DOM SiOC after annealing treatment at 800 °C in air. The SEM image of the material shows partial collapse, but the general macroporous structure is reserved.
Compared with Fig. 1c, the macropores are more irregular, ranging from 100 to 400 nm, of which several pores combine into larger pores. The possible reason is the oxidation of the carbon element during sintering of the PS microspheres and SiOC, which serve as a skeleton of the 3DOM structure. This speculation was further confirmed by the following EDX analysis.
Compared with 3DOM SiOC, the 3DOM SiOC/cordierite monoliths (Fig. 1e and f) have a more integrated structure. It is owing to the supporting role of the cordierite to the 3DOM structure, decreasing the degree of collapse. The interaction of the cordierite and 3DOM structure improves the performance of both.
The SEM analyses were performed on different regions of the 3DOM SiOC/cordierite, Fig. 1e and f show the morphology of the corner and the center of the wall, respectively. From the images, we can see that the layer thickness is not homogeneous, being thicker at the corner of the walls, but only one layer at the center of the walls. The difference in thickness is caused by the fluid dynamic phenomena during the blowing step of making the template on walls and the filling process.24,25 Generally speaking, the adherence force of the corners is stronger than that of the centers, which may be another reason for the different layer thicknesses.
After several times of the immersion–sintering process, the LCO supported 3DOM SiOC/cordierite monoliths were investigated by SEM. Fig. 1g and h show that after several times of sintering, 3DOM materials collapsed locally. Macropores combined to form larger pores, whose sizes range from 200 to 600 nm, larger than other 3DOM samples, and the shape of the macropores show more irregular roundness. But the materials still present the 3DOM structure. The SEM images demonstrate excellent properties for the catalysts. From the images, we can see that the catalyst particles are uniformly distributed on the 3DOM walls, and their sizes range from 30 to 50 nm, much smaller than the catalyst particles made by the sol–gel method (the corresponding SEM images are shown in Fig. S1†), indicating that the 3DOM structure plays an important role in restraining the aggregation of catalyst particles, which is good for improving the contact condition between catalysts and soot. In addition, from the image we can see a connectivity structure of the 3DOM structure, which is convenient for gas flow, making it easier for gas diffusion into soot and the catalysts.26 The phase structure of the catalysts was also demonstrated by XRD and FTIR, as shown in Fig. S2 and S3.†
The EDX analysis of 3DOM SiOC (Fig. 2) confirmed the reason for the collapse of the 3DOM structure when annealed in air. The EDX analysis shows that the main elements of the two materials (before and after annealing in air) are still Si, O and C. However, after the annealing process, the content of the carbon element declined obviously. The relative average atomic ratio was Si: 32 to O: 36 to C: 31 before the annealing process (Fig. 2a), and Si: 61 to O: 26 to C: 12 after the annealing process (Fig. 2b). The decline of the carbon element led to the change of 3DOM materials’ structure. There is still some of the carbon element after the annealing process, which is from the skeleton of the SiOC amorphous structure.
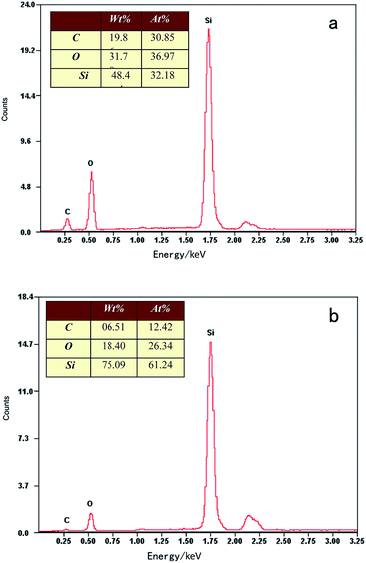 | ||
| Fig. 2 EDX analysis of 3DOM SiOC: (a) 3DOM SiOC sintered at 800 °C in Ar atmosphere, (b) 3DOM SiOC annealing treatment at 800 °C in air. | ||
Fig. 3 shows the EDX analysis of LCO/3DOM SiOC/cordierite. From the EDX analysis we can see that although the main elements are Si, O and C, there are still La and Co elements remaining in their corresponding positions. It precisely demonstrates that the catalysts had been immersed into the interior of the macroporous structure after several times of the immersion–sintering process. The active elements kept the approximate stoichiometric ratio. It demonstrates that LCO was successfully coated onto 3DOM SiOC/cordierite.
In order to further exact the morphology and the properties of the pores (Fig. S4 and S5†) of the 3DOM structure, pore volumes, pore sizes and BET surface areas of the samples were measured.
The results are summarized in Table 2. The results of the pore-size distribution are precisely the same as the results of the SEM. This kind of pore size is suitable for soot and catalysts to pass, and catalysts to load. And the BET results clearly demonstrate that the 3DOM structure enlarged the specific surface area significantly, which has an important effect on the contact condition with soot and diffusion of gas, thus improving the catalyst activity.
| Sample | 3DOM SiOC | LCO/3DOM | Cordierite monolith |
|---|---|---|---|
| Total pore volume (mL g−1) | 0.083 | 0.076 | — |
| Average pore diameter (nm) | 53.98 | 96.22 | — |
| BET surface area (m2 g−1) | 61.33 | 33.69 | 14.44 |
3.2. The reducibility of LCO
H2-TPR experiments were conducted to investigate the reducibility of the samples, and their profiles are illustrated in Fig. 4. For the two samples, there are two reduction peaks: the first reduction peak was due to the reduction of surface Co3+ to Co2+ and the adsorbed oxygen species, the second reduction peak was due to the reduction of Co2+ to Co0.27,28 The two reduction peaks of LCO/3DOM SiOC (435 °C and 595 °C) were much lower than those (468 °C and 633 °C) of LCO, indicating that the reduction of LCO/3DOM SiOC exhibited better reducibility than LCO due to the 3DOM structure. And the reduction peaks of LCO/3DOM SiOC/cordierite are similar to those of 3DOM LCO.293.3. The 3DOM structure for soot combustion
In order to assess the activity of the 3DOM structure for soot combustion, a soot and SiO2 mixture (SiO2 to soot, 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, mass ratio, tight contact, Sample A), a soot and SiOC mixture (SiOC to soot, 9
1, mass ratio, tight contact, Sample A), a soot and SiOC mixture (SiOC to soot, 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, mass radio, tight contact, Sample B), and soot supported on 3DOM SiOC (Sample C) were tested by TG-DSC. The combustion curves are shown in Fig. 5 and the corresponding temperatures are listed in Table 3. Sample A and Sample B have similar characteristic temperatures, which clearly demonstrates that the carbon element remaining in SiOC has no obvious effects on the combustion temperature of soot. Compared with Sample A, T10, T50, Tm and T90 of Sample C are decreased by 27 °C, 36 °C, 37 °C and 52 °C, respectively. In conclusion, the 3DOM structure has excellent qualities for soot combustion, listed as follows: first, the 3DOM structure has a fine connectivity which is convenient for gas flow. Secondly, the 3DOM structure enlarges the specific surface area efficiently. The two features above contribute to diffusion of gas into the soot.26 Thirdly, the 3DOM structure has a uniform pore size which assists soot in spreading. All of these excellent properties of the 3DOM structure add to the unique performance in lowering of the combustion temperature.
1, mass radio, tight contact, Sample B), and soot supported on 3DOM SiOC (Sample C) were tested by TG-DSC. The combustion curves are shown in Fig. 5 and the corresponding temperatures are listed in Table 3. Sample A and Sample B have similar characteristic temperatures, which clearly demonstrates that the carbon element remaining in SiOC has no obvious effects on the combustion temperature of soot. Compared with Sample A, T10, T50, Tm and T90 of Sample C are decreased by 27 °C, 36 °C, 37 °C and 52 °C, respectively. In conclusion, the 3DOM structure has excellent qualities for soot combustion, listed as follows: first, the 3DOM structure has a fine connectivity which is convenient for gas flow. Secondly, the 3DOM structure enlarges the specific surface area efficiently. The two features above contribute to diffusion of gas into the soot.26 Thirdly, the 3DOM structure has a uniform pore size which assists soot in spreading. All of these excellent properties of the 3DOM structure add to the unique performance in lowering of the combustion temperature.
 | ||
| Fig. 5 TG-DSC curves of (a) solid curves: the soot and SiO2 mixture, (b) short dots: the soot and SiOC mixture, (c) dashed curves: soot supported on 3DOM SiOC. | ||
3.4. LCO supported on a 3DOM structure for soot combustion
At present, we further studied the features of LCO/3DOM SiOC/cordierite for soot combustion. As Fig. 6, S6† and Table 4 show, compared with soot over 3DOM SiOC/cordierite and SiO2, T10 of soot over LCO/3DOM SiOC/cordierite is decreased by 50 °C and 77 °C, respectively, T50 is decreased by 21 °C and 57 °C, Tm is decreased by 10 °C and 47 °C, and T90 is decreased by 0 °C and 52 °C. They all showed an obvious advantage of 3DOM SiOC/cordierite over SiO2 especially in the middle and later stages of soot combustion. The explanation for this phenomenon is that in the process of non-catalytic combustion, the initiatory combustion of soot begins in a partial area, and then gradually diffuses to the whole area with the heat temperature rising. Once the soot combustion began, a huge amount of energy was released and transferred, which could partly fulfil the activation energy for soot combustion. At this stage, the diffusion of gas to the soot surface is another important factor influencing the soot–catalyst–gas three-phase reaction. The 3DOM structure has a larger specific surface area and better connectivity, so gas could more easily diffuse to the surface of soot for soot combustion, therefore, as Fig. 6 depicts, in the early stage, the combustion temperature of a and b are similar, in the middle and later stage, the 3DOM structure could reduce the temperature of soot combustion efficiently, and this phenomenon obviously coincided with the rising of the temperature. As for catalytic combustion, there are three kinds of active oxygen species on the surface of LCO,30 and LCO/3DOM SiOC/cordierite could reduce the combustion temperature greatly. As a result, the combustion of soot was dominated by both the 3DOM structure and catalysts in the early and middle stages, and dominated by the 3DOM structure in the later stage. | ||
| Fig. 6 Soot conversion curves over different supports: (a) SiO2, (b) 3DOM SiOC/cordierite, (c) LCO/3DOM SiOC/cordierite. | ||
| Supporting samples | T10 /°C | T50 /°C | Tm /°C | T90 /°C |
|---|---|---|---|---|
| SiO2 | 470 | 535 | 551 | 570 |
| 3DOM SiOC/cordierite | 443 | 499 | 514 | 518 |
| LCO/3DOM SiOC/cordierite | 393 | 478 | 504 | 518 |
3.5. Contact conditions between LCO and soot on the 3DOM structure
The catalysts supported on 3DOM SiOC/cordierite had shown excellent properties for soot combustion. LCO catalyst particles for soot combustion (loose contact) were used to further prove the advantage that the 3DOM structure has on the contact condition between catalysts and soot. LCO/3DOM SiOC/cordierite was immersed into the carbon black–ethanol solution to simulate the practical situation (3DOM contact). From Fig. 7, compared with loose contact, T10, T50 and T90 of 3DOM contact decrease by 36 °C, 37 °C and 37 °C respectively. As J. Xu et al.29 reported, compared with nanoparticles of LCO, the T50 of 3DOM LCO is lower by 15 °C, in this study, the T50 of LCO/3DOM SiOC/cordierite is lower by 37 °C, indicating better properties for soot combustion. We can draw the conclusion that the 3DOM structure improved the contact conditions between soot and catalysts for the excellent structure properties of the 3DOM structure, and thus the soot combustion temperature is further reduced.4. Conclusions
Three-dimensionally ordered macroporous (3DOM) SiOC/cordierite has been successfully fabricated. 3DOM SiOC/cordierite has excellent connectivity (convenient for gas flow and diffusion onto a solid surface), a large specific surface area (much larger than that of a cordierite monolith), and an applicable pore size (100 to 400 nm). 3DOM SiOC/cordierite can significantly reduce the soot combustion temperature for these excellent properties of the 3DOM structure, T10, T50, Tm and T90 of soot over 3DOM SiOC/cordierite decrease by 27 °C, 36 °C, 37 °C and 52 °C, respectively.Perovskite-type LaCoO3 (LCO) mixed metal oxides prepared by the sol–gel method are loaded onto 3DOM SiOC/cordierite, LCO/3DOM SiOC/cordierite could further reduce the combustion temperature of soot, avoiding the back-pressure rising effectively. Compared with 3DOM SiOC/cordierite and bare soot, T10 of soot over catalysts supported on 3DOM SiOC/cordierite decreased by 50 °C and 77 °C, respectively, T50 decreased by 21 °C and 57 °C, Tm decreased by 10 °C and 47 °C, and T90 decreased by 0 °C and 52 °C. And the contact condition between soot and LCO/3DOM SiOC/cordierite is better than that of loose contact of soot and catalysts.
Acknowledgements
This study was supported by the Natural Science Foundation of Tianjin (12JCQNJC05700) and the Natural Science Foundation of Hebei Province of China (E2014202194).References
- J. Neeft, M. Makkee and J. A. Moulijn, Fuel Process. Technol., 1996, 47, 1–69 CrossRef CAS.
- B. Giechaskiel, B. Alföldy and Y. Drossinos, J. Aerosol Sci., 2009, 40, 639–651 CrossRef CAS PubMed.
- J. Adler, Int. J. Appl. Ceram. Technol., 2005, 2, 429–439 CrossRef CAS PubMed.
- J. Yang, M. Stewart, G. Maupin, D. Herling and A. Zelenyuk, Chem. Eng. Sci., 2009, 64, 1625–1634 CrossRef CAS PubMed.
- M. Dhakad, T. Mitshuhashi, S. Rayalu, P. Doggali, S. Bakardjiva, J. Subrt, D. Fino, H. Haneda and N. Labhsetwar, Catal. Today, 2008, 132, 188–193 CrossRef CAS PubMed.
- A. Mishra and R. Prasad, Int. J. Appl. Eng. Res., 2014, 9, 9–16 Search PubMed.
- S. Fang, L. Wang, Z. Sun, N. Feng, C. Shen, P. Lin, H. Wan and G. Guan, Catal. Commun., 2014, 49, 15–19 CrossRef CAS PubMed.
- N. Nejar, J. M. Garcia-Cortes, C. Salinas-Martínez de Lecea and M. J. Illán-Gómez, Catal. Commun., 2005, 6, 263–267 CrossRef CAS PubMed.
- W. F. Shanggua, Y. Teraoka and S. Kagawa, Appl. Catal., B, 1998, 16, 149–154 CrossRef.
- I. S. Pieta, M. García-Diéguez, C. Herrera, M. A. Larrubia and L. J. Alemany, J. Catal., 2010, 270, 256–267 CrossRef CAS PubMed.
- H. Shimokawa, H. Kusaba, H. Einaga and Y. Teraoka, Catal. Today, 2008, 139, 8–14 CrossRef CAS PubMed.
- D. D. Jayaseelan, W. E. Lee, D. Amutharani, S. zhang, K. Yoshida and H. Kita, J. Am. Ceram. Soc., 2007, 90, 1603–1606 CrossRef CAS PubMed.
- J. Neeft, M. Makkee and J. A. Moulijn, Appl. Catal., B, 1996, 8, 57–78 CrossRef CAS.
- J. Neeft, O. P. van Pruissen, M. Makkee and J. A. Moulijn, Appl. Catal., B, 1997, 12, 21–31 CrossRef CAS.
- L. Li, X. Shen, P. Wang, X. Meng and F. Song, Appl. Surf. Sci., 2011, 257, 9519–9524 CrossRef CAS PubMed.
- A. Stein, B. E. Wilson and S. G. Rudisill, Chem. Soc. Rev., 2013, 42, 2763–2803 RSC.
- G. Zhang, Z. Zhao, J. Xu, J. Zheng, J. Liu, G. Jiang, A. Duan and H. He, Appl. Catal., B, 2011, 107, 302–315 CrossRef CAS PubMed.
- J. Zheng, J. Liu, Z. Zhao, J. Xu, A. Duan and G. Jiang, Catal. Today, 2012, 191, 146–153 CrossRef CAS PubMed.
- M. Sadakane, T. Asanuma, J. Kubo and W. Ueda, Chem. Mater., 2005, 17, 3546–3551 CrossRef CAS.
- Y. Wei, J. Liu, Z. Zhao, A. Duan, G. Jiang, C. Xu, J. Gao, H. He and X. Wang, Energy Environ. Sci., 2011, 4, 2959–2970 CAS.
- J. Xu, J. Liu, Z. Zhao, J. Zheng, G. Zhang, A. Duan and G. Jiang, Catal. Today, 2010, 153, 136–142 CrossRef CAS PubMed.
- C. Gong, C. Song, Y. Pei, G. Lv and G. Fan, Ind. Eng. Chem. Res., 2008, 47, 4374–4378 CrossRef CAS.
- B. T. Holland, C. F. Blanford, T. Do and A. Stein, Chem. Mater., 1999, 11, 795–805 CrossRef CAS.
- J. M. Zamaro, M. A. Ulla and E. E. Miró, Chem. Eng. J., 2005, 106, 25–33 CrossRef CAS PubMed.
- E. D. Banús, V. G. Milt, E. E. Miró and M. A. Ulla, Appl. Catal., B, 2013, 132, 479–486 CrossRef PubMed.
- B. R. Stanmore, J. F. Brilhac and P. Gilot, Carbon, 2001, 39, 2247–2268 CrossRef CAS.
- T. Nakamura, G. Petzow and L. J. Gauckler, Mater. Res. Bull., 1979, 14, 649–659 CrossRef CAS.
- B. Sextori, A. Hughes and T. Turney, J. Catal., 1986, 97, 390–406 CrossRef.
- J. Xu, J. Liu, Z. Zhao, C. Xu, J. Zheng, A. Duan and G. Jiang, J. Catal., 2011, 282, 1–12 CrossRef CAS PubMed.
- H. Liang, Y. Hong, C. Zhu, S. Li, Y. Chen, Z. Liu and D. Ye, Catal. Today, 2013, 201, 98–102 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra05067g |
| This journal is © The Royal Society of Chemistry 2015 |