In situ growth of ZnO nanorod arrays on cotton cloth for the removal of uranium(vi)†
Abstract
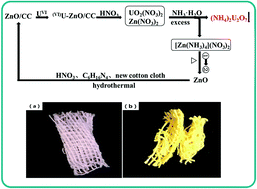
In situ growth of ZnO nanorod arrays on cotton cloth (ZnO/CC) was proposed to remove uranium(VI) from aqueous solutions. The as-prepared adsorbent is easy to separate from the reaction medium after adsorption. The effect factors for uranium adsorption, such as solution pH, initial U(VI) concentration, contact time, and temperature have been systematically investigated. The maximum adsorption capacity of uranium(VI) which was calculated by the Langmuir model at pH = 5.0 and T = 298 K is 431.03 mg g−1, exhibiting excellent uranium adsorption properties. It was observed that the kinetic data fit well with a pseudo-second-order kinetic model indicating that the rate-limiting step of the adsorption process is chemical adsorption. Moreover, thermodynamic parameters [ΔH0 = 20.26 kJ mol−1 ΔG0 = −5.66 kJ mol−1 (298 K) ΔS0 = 86.98 J mol−1 K−1] reveal that the uranium adsorption is endothermic and spontaneous. Therefore, the ZnO/CC is a potential adsorbent for recovery of uranium(VI) from aqueous solutions.

 Please wait while we load your content...
Please wait while we load your content...