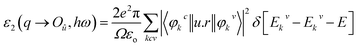Ba7(BO3)3GeO4X (X = Cl, Br): borogermanate halides with rigid GeO4 tetrahedra and flexible XBa6 octahedra†
Ming Wenab,
Zhipeng Lianc,
Hongping Wu*a,
Xin Suab,
Qingfeng Yanc,
Juanjuan Luab,
Zhihua Yang*a and
Shilie Pan*a
aKey Laboratory of Functional Materials and Devices for Special Environments of CAS, Xinjiang Key Laboratory of Electronic Information Materials and Devices, Xinjiang Technical Institute of Physics & Chemistry of CAS, 40-1 South Beijing Road, Urumqi 830011, China. E-mail: wuhp@ms.xjb.ac.cn.; slpan@ms.xjb.ac.cn; Fax: +86-991-3838957; Tel: +86-991-3674558
bUniversity of Chinese Academy of Sciences, Beijing 100049, China
cDepartment of Chemistry, Tsinghua University, Beijing, 100084, China
First published on 28th May 2015
Abstract
The first borogermanate halides, Ba7(BO3)3GeO4X (X = Cl, Br), have been synthesized using a high temperature solution method. Ba7(BO3)3GeO4X (X = Cl, Br) are isostructural and crystallize in the orthorhombic space group Pbam (no. 55). They feature a Ba-based 3D framework, while isolated GeO4 tetrahedra and BO3 triangles fill in the space created by the framework. Significantly, Ba7(BO3)3GeO4X (X = Cl, Br) possess a similar formula to the borosilicate halides, Ba7(BO3)3SiO4X (X = Cl, Br), but they are not isostructural. An interpretation for the structural transformation between them is demonstrated here, considering rigid GeO4 tetrahedra and flexible XBa6 (X = Cl, Br) octahedra. IR spectroscopy, UV-Vis-NIR diffuse reflectance spectroscopy and first-principles calculations have been carried out on the title compounds.
Introduction
Borates have been widely investigated owing to their rich chemical structures.1–9 In particular, borates with planar BO3 units have attracted considerable attention, because the π-delocalization of the BO3 group is beneficial for a strong second-harmonic generation (SHG) response and large birefringence based on anionic group theory. A statistical analysis of the fundamental building blocks of borate, carried out by P. Becker,10 indicates that borates with isolated BO3 groups will be found in systems with appropriate cation/boron ratios (>1). Thus, in the alkaline-earth borates, compounds with the formula M7(BO3)3F5 (M = Ba or Sr), with a large cation/boron ratio, have attracted the interest of scientists.In the M7(BO3)3F5 (M = Ba or Sr) system, two compounds, Ba7(BO3)3F5 (ref. 11) and Ba3Sr4(BO3)3F5 (ref. 11), were first identified by Keszler et al. in 1994. In 2008, Zhang’s group reinvestigated the crystal structure of Ba3Sr4(BO3)3F5 and grew large crystals using the top-seeded solution growth method, which showed an SHG intensity about 0.5 times as large as that of KH2PO4 (KDP).12 Then, in 2012, Bekker et al. systematically studied the M7(BO3)3F5 (M = Ba or Sr) system. They found that a disordered state between (4F)4− and (BO3F)4− exists in the system and a series compounds of Ba7(BO3)4−xF2+3x and Ba4−xSr3+x(BO3)4−yF2+3y (ref. 13 and 14) were identified. However, the existence of disorder in the crystal structure is not favorable for excellent physical or chemical properties.
The SiO4 tetrahedra possess the same charge as (4F)4− or (BO3F)4− and can be introduced into borate to replace them, which may solve the disorder in the crystal structure and improve the properties. Inspired by this, the compounds Ba7(BO3)3SiO4X (X = Cl, Br)15 have been synthesized by our group, in which the structural disorder disappears. Ba7(BO3)3SiO4X (X = Cl, Br) have a SHG response as strong as that of KDP. However, as is well-known, the high reaction temperature and high viscosity of the silicate are not conducive to the growth of crystals. Compared to silicates, germanates have a lower reaction temperature, and in previous research, many alkaline and alkaline-earth metal borogermanates have been reported.16–20 In addition, the Ge and Si elements are in the same main group, and possess similar coordination environments. Guided by these ideas, the first borogermanate halides, Ba7(BO3)3GeO4X (X = Cl, Br), have been successfully synthesized. Owing to the large cation/boron ratio (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), the BO3 triangles and GeO4 tetrahedra are all isolated, which is quite rare in inorganic borogermanates.21 It is worth noting that although the Ge and Si atoms possess similarity, Ba7(BO3)3GeO4X (X = Cl, Br) and Ba7(BO3)3SiO4X (X = Cl, Br) crystallize in different space groups, centrosymmetric Pbam (no. 55) and polar P63mc (no. 186), respectively.
3), the BO3 triangles and GeO4 tetrahedra are all isolated, which is quite rare in inorganic borogermanates.21 It is worth noting that although the Ge and Si atoms possess similarity, Ba7(BO3)3GeO4X (X = Cl, Br) and Ba7(BO3)3SiO4X (X = Cl, Br) crystallize in different space groups, centrosymmetric Pbam (no. 55) and polar P63mc (no. 186), respectively.
Thus, we have investigated the substitution of Ge for Si in other reported borates, determined from single crystal X-ray diffraction structures.17,18,22–28 We found that most of the compounds are isostructural (Table S1 in the ESI†). In these borates,17,18,22–28 we define a parameter of R (O/M, M = Si or Ge), which represents the mole ratio of the O element and the substitution element. In general, a higher value of R means a lower mole ratio of the substituted element in the compounds, which results in a smaller change due to the substitution and more likely results in isostructural substitution. Therefore, when R is higher than 3, all the compounds are isostructural.17,23–28 It is unusual that Ba7(BO3)3GeO4X (X = Cl, Br) and Ba7(BO3)3SiO4X (X = Cl, Br) are not isostructural, with a quite high R value of 13. The structural transformation between Ba7(BO3)3GeO4X (X = Cl, Br) and Ba7(BO3)3SiO4X (X = Cl, Br) has been discussed in detail in this paper. We have also reported the spectra and the first-principles calculations of the title compounds.
Experimental
Solid-state synthesis
Polycrystalline samples of Ba7(BO3)3GeO4X (X = Cl, Br) were synthesized via conventional solid-state reactions. Stoichiometric mixtures of BaCO3, BaCl2, GeO2, and B2O3 at a molar ratio of 6.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5
0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 for Ba7(BO3)3GeO4Cl, and BaCO3, BaBr2, GeO2, and B2O3 at a molar ratio of 6.5
1.5 for Ba7(BO3)3GeO4Cl, and BaCO3, BaBr2, GeO2, and B2O3 at a molar ratio of 6.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5
0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 for Ba7(BO3)3GeO4Br, were ground and packed into alumina crucibles. The raw materials were preheated at 600 °C for 14 h. The mixtures were heated to 900 °C for Ba7(BO3)3GeO4Cl and 950 °C for Ba7(BO3)3GeO4Br, then held for 4 days, and finally cooled to room temperature. During the sintering processes the samples were ground thoroughly. The purity of the samples was confirmed by powder X-ray diffraction (XRD), which agrees well with the theoretical patterns of the compounds (Fig. S1 in the ESI†).
1.5 for Ba7(BO3)3GeO4Br, were ground and packed into alumina crucibles. The raw materials were preheated at 600 °C for 14 h. The mixtures were heated to 900 °C for Ba7(BO3)3GeO4Cl and 950 °C for Ba7(BO3)3GeO4Br, then held for 4 days, and finally cooled to room temperature. During the sintering processes the samples were ground thoroughly. The purity of the samples was confirmed by powder X-ray diffraction (XRD), which agrees well with the theoretical patterns of the compounds (Fig. S1 in the ESI†).
Crystal growth
Single crystals of Ba7(BO3)3GeO4X (X = Cl, Br) were grown from the high temperature solutions using NaCl–LiCl, NaBr–LiBr and B2O3 as flux systems. The solutions were obtained in platinum crucibles by melting mixtures of BaCO3, GeO2, B2O3, NaCl and LiCl at a molar ratio of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5
2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 for Ba7GeB3O13Cl, and BaCO3, GeO2, B2O3, NaBr and LiBr at a molar ratio of 7
5 for Ba7GeB3O13Cl, and BaCO3, GeO2, B2O3, NaBr and LiBr at a molar ratio of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5
2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 for Ba7GeB3O13Br. The solutions were heated to 900 °C, maintained at this temperature for 10 h, then slowly cooled to 700 °C at a rate of 3 °C h−1 and finally cooled down to room temperature at a rate of 10 °C h−1. Crystals of Ba7(BO3)3GeO4X (X = Cl, Br) were obtained, which were used for the single-crystal X-ray diffraction analysis.
4 for Ba7GeB3O13Br. The solutions were heated to 900 °C, maintained at this temperature for 10 h, then slowly cooled to 700 °C at a rate of 3 °C h−1 and finally cooled down to room temperature at a rate of 10 °C h−1. Crystals of Ba7(BO3)3GeO4X (X = Cl, Br) were obtained, which were used for the single-crystal X-ray diffraction analysis.
Structure determination
The crystal structures of Ba7(BO3)3GeO4X (X = Cl, Br) were determined using a Bruker SMART APEX II CCD diffractometer with monochromatic Mo Kα radiation at 293(2) K, and were further integrated with the SAINT program.29 The calculations were completed using programs from the SHELXTL crystallographic software package, while the structures were determined using direct methods with SHELXS-97. Final least-squares refinements are on Fo2 with data having Fo2 ≥ 2σ(Fo2). PLATON has been used to check for missing symmetry.30 Crystal data and structure refinement information are shown in Table 1, and the final refined atomic positions and isotropic thermal parameters are displayed in Table S2 in the ESI.†| a R1 = ∑||Fo| − |Fc||/∑|Fo| and wR2 = [∑w(Fo2 − Fc2)2/∑wFo4]1/2 for Fo2 > 2σ(Fo2). | |||
|---|---|---|---|
| Empirical formula | Ba7(BO3)3GeO4Cl | Ba7(BO3)3GeO4Br | |
| Temperature | 296(2) K | ||
| Wavelength | 0.71073 Å | ||
| Crystal system | Orthorhombic | ||
| Space group | Pbam | ||
| Formula weight | 1309.85 | 1354.31 | |
| a (Å) | 20.353(18) | 20.381(8) | |
| b (Å) | 7.416(6) | 7.477(3) | |
| c (Å) | 11.123(10) | 11.175(4) | |
| Z, volume (Å3) | 4, 1679(3) | 4, 1702.9(11) | |
| ρcalcd (mg m−3) | 5.183 | 5.283 | |
| μ (mm) | 18.121 | 20.054 | |
| R(int) | 0.0776 | 0.0651 | |
| Goodness-of-fit on F2 | 1.037 | 1.043 | |
| Final R indices [Fo2 > 2σ(Fo2)]a | R1 = 0.0351 | R1 = 0.0315 | |
| wR2 = 0.0612 | wR2 = 0.0585 | ||
| R indices (all data)a | R1 = 0.0545 | R1 = 0.0426 | |
| wR2 = 0.0675 | wR2 = 0.0630 | ||
| Extinction coefficient | 0.00061(4) | 0.00207(7) | |
| Largest diff. peak and hole (e Å−3) | 2.403 and −2.209 | 2.158 and −2.070 | |
Infrared and UV-Vis-NIR diffuse reflectance spectroscopy
Infrared spectroscopy in the range of 400–4000 cm−1 has been performed on a Shimadzu IR Affinity-1 Fourier transform infrared spectrometer. UV-Vis-NIR diffuse reflectance data have been obtained on a Solid Spec-3700DUV spectrophotometer with a range from 190 to 2600 nm. Polytetrafluoroethylene was used as a standard. Finally, we converted the reflectance spectra to absorbance using the function, F(R) = (1 − R)2/2R. R represents the reflectance while F(R) represents the Kubelka–Munk remission function.31Numerical calculation details
The electronic structure calculations were performed using a plane-wave basis set and pseudopotentials within density functional theory (DFT), implemented in the total-energy module CASTEP.32 The exchange and correlation effects were treated with Perdew–Burke–Ernzerhof (PBE) in the generalized gradient approximation (GGA).33–35 The interactions between the ionic cores and the electrons were described by ultrasoft pseudopotentials. The following orbital electrons were treated as valence electrons: Ba 5s25p66s25d0, Ge 4s24p2, B 2s22p1, O 2s22p4, Cl 3s23p5 and Br 4s24p5. The number of plane waves included in the basis were determined by a cutoff energy of 340 eV, and the numerical integration of the Brillouin zone was performed using a 4 × 4 × 3 Monkhorst–Pack scheme36 k-point grid sampling for Ba7(BO3)3GeO4Cl and Ba7(BO3)3GeO4Br. Our test showed that these computational parameters ensure a good convergence in the present studies.The linear optical response properties of Ba7(BO3)3GeO4X (X = Cl, Br) were examined by calculating the complex dielectric function ε(ω) = ε1(ω) + iε2(ω). The imaginary part of the dielectric function ε2 is given in the following equation:37
The real part ε1(ω) can be obtained from the imaginary part ε2(ω) by the Kramers–Kronig transformation. All the other optical constants, such as the absorption spectrum, refractive index, and reflectivity are derived from ε1(ω) and ε2(ω).
Results and discussion
Structure
Both of the title compounds crystallize in an orthorhombic space group, Pbam (no. 55). As Ba7(BO3)3GeO4X (X = Cl, Br) are isostructural, only the structure of Ba7(BO3)3GeO4Br is described in detail here. There are six unique Ba sites, two unique B sites, one unique Ge site, one unique Br site and eight unique O sites in the asymmetric unit (Table S2 in the ESI†). In the structure of Ba7(BO3)3GeO4Br, BaOn (n = 8, 9) and BaO7Br2 polyhedra are interconnected by shared oxygen atoms to form the 3D framework (Fig. 1a), while isolated GeO4 tetrahedra and BO3 triangles fill in the space created by the framework (Fig. 1b). | ||
| Fig. 1 The structure of Ba7(BO3)3GeO4Br. (a) Ba-based 3D framework. (b) 3D network of Ba7(BO3)3GeO4Br. All Ba–O and Ba–Br bonds have been omitted for clarity. | ||
Selected bond distances and angles for Ba7(BO3)3GeO4X (X = Cl, Br) are presented in Fig. S3 in the ESI.† The Ba–O, B–O, Ge–O and Ba–Br distances vary over 2.567(7)–3.217(5) Å, 1.362(12)–1.391(9) Å, 1.737(7)–1.766(5) Å and 3.1424(15)–3.7463(13) Å, respectively. These values are observed in other reported compounds.16a,38
Comparing the structure of Ba7(BO3)3GeO4X (X = Cl, Br) with Ba7(BO3)3SiO4X (X = Cl, Br)
Ba7(BO3)3SiO4X (X = Cl, Br) are isostructural and crystallize in the hexagonal P63mc space group, while Ba7(BO3)3GeO4X (X = Cl, Br) crystallize in orthorhombic Pbam. Three unique Ba atoms, one unique B atom, one unique Si atom, four unique O atoms and one unique Br atom are in the asymmetric unit of Ba7(BO3)3SiO4Br. The structure of Ba7(BO3)3SiO4Br is composed of a 3D Ba(3)-based framework with tunnels viewed along the c axis, in which the BrBa(2)3 chains reside (Fig. 2a). In addition, ∞1[Ba(1)O6(SiO4)] single chains and isolated BO3 triangles fill in the framework to form its 3D network structure (Fig. 2b and c). In the structure, the ∞1[Ba(1)O6(SiO4)] single chains consist of isolated SiO4 tetrahedra and Ba(1)O10 polyhedra. | ||
| Fig. 2 The structure of Ba7(BO3)3SiO4Br. (a) ∞1[BrBa3]. (b) Ba(3)-based 3D framework. (c) 3D network of Ba7(BO3)3SiO4Br. All Ba–O bonds have been omitted for clarity. | ||
Similarly, the structure of Ba7(BO3)3GeO4Br is composed of a 3D Ba(1, 4, 5)-based framework with tunnels viewed along b axis, in which the BrBa(2, 6)3 chains are located (Fig. 3a). In addition, ∞1{[Ba(3)O4(GeO4)]2} double chains and isolated BO3 triangles fill in the framework to form its 3D network structure (Fig. 3b and c). The ∞1{[Ba(3)O4(GeO4)]2} double chains are built of isolated GeO4 tetrahedra and Ba(3)O10 polyhedra.
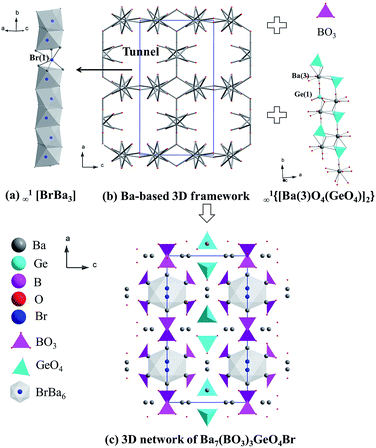 | ||
| Fig. 3 The structure of Ba7(BO3)3GeO4Br. (a) ∞1[BrBa3]. (b) Ba-based 3D framework. (c) 3D network of Ba7(BO3)3GeO4Br. All Ba–O bonds have been omitted for clarity. | ||
Accordingly, the structural transformation between Ba7(BO3)3SiO4Br and Ba7(BO3)3GeO4Br will be discussed from the following two perspectives: (A) the arrangements of chains and (B) the Ba-based 3D frameworks.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Si or Ba
Si or Ba![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ge molar ratio of 7
Ge molar ratio of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, but the Ba atoms are arranged in different configurations. In the structure of Ba7(BO3)3SiO4Br, the BrBa6 octahedra are in the center of six neighboring SiO4 tetrahedra and the SiO4 tetrahedra connect with the Ba(3) atoms to form a single chain viewed along the c axis (Fig. 5a). From Fig. 5b, we can also observe that every six Ba atoms are arranged around one SiO4 tetrahedron. The rigid SiO4 tetrahedra have pushed the Ba(2) atoms to the Br atoms, which makes the six Ba atoms have a pattern like an equilateral triangle (Fig. 5b). Six equilateral triangles constitute a hexagonal configuration template. This suggests that Ba7(BO3)3SiO4Br crystallizes in the hexagonal crystal system. Similarly, in the structure of Ba7(BO3)3GeO4Br, the BrBa6 octahedra are located at the center of the SiO4 tetrahedra and the Ba atoms lie around the SiO4 tetrahedra (Fig. 5c and d). Owing to the squeeze from the SiO4 tetrahedra to the Ba(2) and Ba(6) atoms, the Ba atoms form a pattern analogous to a rectangle. Four rectangles compose a bigger one, which can become a rectangular template (Fig. 5d). This implies that Ba7(BO3)3GeO4Br crystallizes in the orthogonal crystal system.
1, but the Ba atoms are arranged in different configurations. In the structure of Ba7(BO3)3SiO4Br, the BrBa6 octahedra are in the center of six neighboring SiO4 tetrahedra and the SiO4 tetrahedra connect with the Ba(3) atoms to form a single chain viewed along the c axis (Fig. 5a). From Fig. 5b, we can also observe that every six Ba atoms are arranged around one SiO4 tetrahedron. The rigid SiO4 tetrahedra have pushed the Ba(2) atoms to the Br atoms, which makes the six Ba atoms have a pattern like an equilateral triangle (Fig. 5b). Six equilateral triangles constitute a hexagonal configuration template. This suggests that Ba7(BO3)3SiO4Br crystallizes in the hexagonal crystal system. Similarly, in the structure of Ba7(BO3)3GeO4Br, the BrBa6 octahedra are located at the center of the SiO4 tetrahedra and the Ba atoms lie around the SiO4 tetrahedra (Fig. 5c and d). Owing to the squeeze from the SiO4 tetrahedra to the Ba(2) and Ba(6) atoms, the Ba atoms form a pattern analogous to a rectangle. Four rectangles compose a bigger one, which can become a rectangular template (Fig. 5d). This implies that Ba7(BO3)3GeO4Br crystallizes in the orthogonal crystal system.
Based on the above analyses, rigid SiO4 tetrahedra and flexible BrBa6 octahedra play key roles in the structural transformation from hexagonal Ba7(BO3)3SiO4Br to orthogonal Ba7(BO3)3GeO4Br.
Infrared and UV-Vis-NIR diffuse reflectance spectroscopy
The infrared spectra of Ba7(BO3)3GeO4X (X = Cl, Br) are quite same. For both of them, the bands that appeared in the ranges of 1350–1450 cm−1 and 1150–1250 cm−1 can be assigned to the asymmetric stretching vibration of the BO3 groups (Fig. S2 in the ESI†). The peaks observed in the region of 733–641 cm−1 are attributed to the out-of-plane bending of BO3. The peaks in the region of 860–900 cm−1 can be assigned to an asymmetrical stretch of Ge–O of the tetrahedral germanium. The absorption bands in the range of 450–600 cm−1 correspond to the bending vibrations of the Ge–O bonds. The infrared spectra further confirm the existence of BO3 triangles and GeO4 tetrahedra, which is consistent with the results obtained from single crystal X-ray structural analyses.The UV-Vis-NIR diffuse reflectance spectra of Ba7(BO3)3GeO4X (X = Cl, Br) are shown in Fig. S3 in the ESI.† They indicate that the experimental optical band gaps of Ba7(BO3)3GeO4Cl and Ba7(BO3)3GeO4Br are 5.28 and 5.31 eV, respectively. The large band gaps of the title compounds suggest that they may be used as optical window materials.
Band structures, density of states and optical properties
The band structures of Ba7(BO3)3GeO4X (X = Cl, Br) are presented in Fig. S4 in the ESI.† The valence band maximum (VBM) and the conduction band minimum (CBM) are located at the Γ point of the Brillouin zone indicating that both of the two compounds belong to direct band-gap semiconductors. The extrapolated experimental optical gaps of 5.28 eV for Ba7(BO3)3GeO4Cl and 5.31 eV for Ba7(BO3)3GeO4Br are slightly larger than the calculated values of 4.93 eV and 4.91 eV, respectively. The underestimation of the band gap is generally due to insufficient accuracy of the exchange correlation energy under DFT methods.39 We can see that the results of our calculations are good and give reasonable explanations for the optical absorption spectra. The highest occupied and lowest unoccupied orbitals determine the route of electronic transitions and the absorption edge. It is shown in Fig. S5 in the ESI† that the absorption edges of Ba7(BO3)3GeO4Cl and Ba7(BO3)3GeO4Br are mainly decided by the O 2p states in the BO3 units.The total and partial densities of states (TDOS and PDOS) are shown in Fig. 6. The top region of the VBs extends in a wide range from −6.7 eV to the VBM. These bands mostly originate from the Ge 4s4p, B 2s2p and O 2s2p states with mixing of the Ba 5s5p and Cl 3p/Br 4p states, especially near the Fermi level. It is worthy of note that strong hybridizations occur among the Ge 4s4p, B 2s2p and O 2s2p states in the range of −6.7 to −0.7 eV. For Ba7(BO3)3GeO4Cl and Ba7(BO3)3GeO4Br, the conduction bands from the CBM to 8.5 eV are derived from the Ba 5d, Ge 4s4p and B 2p states with mixing of the Ba 5s5p, B 2s and O 2s2p states, implying strong interactions in the Ge–O and B–O bonds of the compounds. The valence bands and conduction bands near the gap are dominated by O 2p and Ba 5d, respectively. Accordingly, the absorption spectrum near the UV-visible cut-off wavelength can be assigned as the charge transfers from the O 2p states to the Ba 5d states.
On the basis of the electronic structures, the linear optical properties can be estimated. The imaginary part of the dielectric function ε2 is calculated, and its real part ε1 is determined using the Kramers–Kronig transform, from which the refractive indices and the birefringence Δn were obtained.40 The dispersion curves of the refractive indices are calculated using the formula n2(ω) = ε(ω), displaying a similar optical anisotropy behavior, nx ≈ ny > nz, for Ba7(BO3)3GeO4Cl and Ba7(BO3)3GeO4Br. The birefringence (Δn) versus wavelength is shown in Fig. S6 in the ESI.† It can be seen that the birefringence values of Ba7(BO3)3GeO4Cl and Ba7(BO3)3GeO4Br are about 0.03 with a wavelength of 1064 nm.
Conclusion
The first borogermanate halides, Ba7(BO3)3GeO4X (X = Cl, Br), have been obtained using a high temperature solution method. We have discussed the structural transformation from Ba7(BO3)3SiO4X (X = Cl, Br) to Ba7(BO3)3GeO4X (X = Cl, Br). The unusual structural transformation mainly originates from rigid GeO4 tetrahedra and flexible XBa6 (X = Cl, Br) octahedra. We believe that this interpretation can give new insights into structural transformation. The first-principles calculations show that the calculated band gaps (4.93 eV for Ba7(BO3)3GeO4Cl and 4.91 eV for Ba7(BO3)3GeO4Br) agree well with the experimental ones (5.28 and 5.31 eV). The top of the valence band is dominated by a mixture of B 2p and O 2p states, while the Ba 5d, Ge 4s4p, B 2s and O 2s2p states dominate the bottom of the conduction band.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant nos 21201176, U1303392, 51425206, 51172277, U1129301), 973 Program of China (Grant no. 2014CB648400), the Xinjiang Program of Cultivation of Young Innovative Technical Talents (Grant no. 2014711001), the Western Light of CAS (Grant no. XBBS201214), the Outstanding Young Scientists Project of Chinese Academy of Science, the Xinjiang International Science & Technology Cooperation Program (Grant no. 20146001), the Funds for Creative Cross & Cooperation Teams of CAS, and the Xinjiang Key Laboratory Foundation (Grant no. 2014KL009).Notes and references
- (a) P. Becker, Adv. Mater., 1998, 10, 979 CrossRef CAS; (b) C. T. Chen, B. C. Wu, A. Jiang and G. M. You, Sci. Sin., Ser. B, 1985, 28, 235 Search PubMed; (c) C. T. Chen, Y. C. Wu, A. Jiang, B. C. Wu, G. M. You, R. K. Li and S. J. Lin, J. Opt. Soc. Am. B, 1989, 6, 616 CrossRef CAS; (d) Y. C. Wu, T. Sasaki, S. Nakai, A. Yokotani, H. Tang and C. T. Chen, Appl. Phys. Lett., 1993, 62, 2614 CrossRef CAS PubMed.
- (a) Y. Mori, I. Kuroda, S. Nakajima, T. Sasaki and S. Nakai, Appl. Phys. Lett., 1995, 67, 1818 CrossRef CAS PubMed; (b) C. T. Chen, Y. B. Wang, B. C. Wu, K. C. Wu, W. L. Zeng and L. H. Yu, Nature, 1995, 373, 322 CrossRef CAS PubMed; (c) N. Ye, W. R. Zeng, J. Jiang, B. C. Wu, C. T. Chen, B. H. Feng and X. L. Zhang, J. Opt. Soc. Am. A, 2000, 17, 764 CrossRef CAS; (d) L. Y. Li, G. B. Li, Y. X. Wang, F. H. Liao and J. H. Lin, Chem. Mater., 2005, 17, 4174 CrossRef CAS.
- (a) H. W. Huang, L. J. Liu, S. F. Jin, W. J. Yao, Y. H. Zhang and C. T. Chen, J. Am. Chem. Soc., 2013, 135, 18319 CrossRef CAS PubMed; (b) H. W. Huang, Y. He, Z. S. Lin, L. Kang and Y. H. Zhang, J. Phys. Chem. C, 2013, 117, 22986 CrossRef CAS; (c) R. H. Cong, J. L. Zhu, Y. X. Wang, T. Yang, F. H. Liao, C. Q. Jin and J. H. Lin, CrystEngComm, 2009, 11, 1971 RSC.
- (a) S. G. Zhao, P. F. Gong, S. Y. Luo, S. J. Liu, L. N. Li, M. A. Asghar, T. Khan, M. C. Hong, Z. S. Lin and J. H. Luo, J. Am. Chem. Soc., 2015, 137, 2207 CrossRef CAS PubMed; (b) S. L. Pan, J. P. Smit, M. R. Marvel, E. S. Stampler, J. M. Haag, J. Baek, P. S. Halasyamanin and K. R. Poeppelmeier, J. Solid State Chem., 2008, 181, 2087 CrossRef CAS PubMed; (c) T. Pilz and M. Jansen, Z. Anorg. Allg. Chem., 2011, 637, 1 CrossRef PubMed.
- (a) H. P. Wu, H. W. Yu, Z. H. Yang, X. L. Hou, X. Su, S. L. Pan, K. R. Poeppelmeier and J. M. Rondinelli, J. Am. Chem. Soc., 2013, 135, 4215 CrossRef CAS PubMed; (b) X. W. Zhang, H. W. Yu, H. P. Wu, S. L. Pan, A. Q. Jiao, B. B. Zhang and Z. H. Yang, RSC Adv., 2014, 4, 13195 RSC; (c) H. Y. Li, H. P. Wu, X. Su, H. W. Yu, S. L. Pan, Z. H. Yang, Y. Lu, J. Han and K. R. Poeppelmeier, J. Mater. Chem. C, 2014, 2, 21704 Search PubMed; (d) K. Wu, S. L. Pan and Z. H. Yang, RSC Adv., 2015, 5, 33646 RSC.
- (a) C. Y. Bai, S. J. Han, S. L. Pan, B. B. Zhang, Y. Yang, L. Li and Z. H. Yang, RSC Adv., 2015, 5, 12416 RSC; (b) Y. Yang, S. L. Pan, X. Su, Y. Wang, Z. H. Yang, J. Han, M. Zhang and Z. H. Chen, CrystEngComm, 2014, 16, 1978 RSC; (c) Z. Wang, Q. Jing, M. Zhang, X. Y. Dong, S. L. Pan and Z. H. Yang, RSC Adv., 2014, 4, 54194 RSC; (d) H. P. Wu, H. W. Yu, S. L. Pan, A. Q. Jiao, H. Y. Li, J. Han, K. Wu and S. J. Han, Dalton Trans., 2014, 43, 4886 RSC.
- (a) A. H. Reshak, X. A. Chen, S. Auluck, H. Kamarudin, J. Chyský, A. Wojciechowski and I. V. Kityk, J. Phys. Chem. B, 2013, 117, 14141 CrossRef CAS PubMed; (b) X. A. Chen, H. Yin, X. N. Chang, H. G. Zang and W. Q. Xiao, J. Solid State Chem., 2010, 183, 2910 CrossRef CAS PubMed.
- (a) C. D. McMillen, J. T. Stritzinger and J. W. Kolis, Inorg. Chem., 2012, 51, 3953 CrossRef CAS PubMed; (b) C. Heyward, C. McMillen and J. W. Kolis, Inorg. Chem., 2012, 51, 3956 CrossRef CAS PubMed.
- (a) H. Emme, M. Valldor, R. Pöttgen and H. Huppertz, Chem. Mater., 2005, 17, 2707 CrossRef CAS; (b) J. S. Knyrim, H. Emme, M. Döblinger, O. Oeckler, M. Weil and H. Huppertz, Chem.–Eur. J., 2008, 14, 6149 CrossRef CAS PubMed; (c) M. J. Xia, B. Xu and R. K. Li, J. Cryst. Growth, 2014, 404, 65 CrossRef CAS PubMed.
- P. Becker, Z. Kristallogr., 2001, 216, 523 CrossRef CAS.
- D. A. Keszler, A. Akella, K. I. Schaffers and T. Alekel, Mater. Res. Soc. Symp. Proc., 1994, 329, 15 CrossRef CAS PubMed.
- G. C. Zhang, Z. L. Liu, J. X. Zhang, F. D. Fan, Y. C. Liu and P. Z. Fu, Cryst. Growth Des., 2009, 9, 3139 Search PubMed.
- T. B. Bekker, S. V. Rashchenko, V. V. Bakakin, Y. V. Seryotkin, P. P. Fedorov, A. E. Kokha and S. Y. Stonogaa, CrystEngComm, 2012, 14, 6910 RSC.
- S. V. Rashchenko, T. B. Bekker, V. V. Bakakin, Y. V. Seryotkin, V. S. Shevchenko, A. E. Kokh and S. Y. Stonoga, Cryst. Growth Des., 2012, 12, 2955 CAS.
- X. X. Lin, F. F. Zhang, S. L. Pan, H. W. Yu, F. Y. Zhang, X. Y. Dong, S. J. Han, L. Y. Dong, C. Y. Bai and Z. Wang, J. Mater. Chem. C, 2014, 2, 4257 RSC.
- (a) X. Xu, C. L. Hu, F. Kong, J. H. Zhang and J. G. Mao, Inorg. Chem., 2011, 50, 8861 CrossRef CAS PubMed; (b) D. B. Xiong, J. T. Zhao, H. H. Chen and X. X. Yang, Chem.–Eur. J., 2007, 13, 9862 CrossRef CAS PubMed.
- (a) J. H. Zhang, F. Kong and J. G. Mao, Inorg. Chem., 2011, 50, 3037 CrossRef CAS PubMed; (b) Y. C. Hao, C. L. Hu, X. Xu, F. Kong and J. G. Mao, Inorg. Chem., 2013, 52, 13644 CrossRef CAS PubMed; (c) J. B. Parise and T. E. Gier, Chem. Mater., 1992, 4, 1065 CrossRef CAS.
- Z. E. Lin, J. Zhang and G. Y. Yang, Inorg. Chem., 2003, 42, 1797 CrossRef CAS PubMed.
- (a) H. X. Zhang, J. Zhang, S. T. Zheng, G. M. Wang and G. Y. Yang, Inorg. Chem., 2004, 43, 6148 CrossRef CAS PubMed; (b) D. B. Xiong, H. H. Chen, M. R. Li, X. X. Yang and J. T. Zhao, Inorg. Chem., 2006, 45, 9301 CrossRef CAS PubMed.
- (a) P. Becker, Cryst. Res. Technol., 2001, 2, 119 CrossRef; (b) B. Petermüller, L. L. Petschnig, K. Wurst, G. Heymann and H. Huppertz, Inorg. Chem., 2014, 53, 9722 CrossRef PubMed.
- B. F. Dzhurinskii, A. B. Pobedina, K. K. Palkina and M. G. Komova, Russ. J. Inorg. Chem., 1998, 43, 1488 Search PubMed.
- M. Ihara and F. Kamei, J. Ceram. Soc. Jpn., 1980, 88, 32 CrossRef CAS.
- H. P. Wu, H. W. Yu, S. L. Pan, Z. J. Huang, Z. H. Yang, X. Su and K. R. Poeppelmeier, Angew. Chem., Int. Ed., 2013, 52, 3406 CrossRef CAS PubMed.
- X. Xu, C. L. Hu, F. Kong, J. H. Zhang, J. G. Mao and J. L. Sun, Inorg. Chem., 2013, 52, 5831 CrossRef CAS PubMed.
- T. Berger and K. J. Range, Z. Naturforsch., B: J. Chem. Sci., 1996, 51, 172 CAS.
- C. Heyward, C. D. McMillen and J. J. Kolis, Solid State Chem., 2013, 203, 166 CrossRef CAS PubMed.
- V. R. Samygina, E. A. Genkina, B. A. Maksimov and N. I. Leonyuk, Kristallografiya, 1993, 38, 61 CAS.
- A. A. Kaminskii, B. V. Mill, E. L. Belokoneva and A. V. Butashin, Izv. Akad. Nauk SSSR, Energ. Transp., 1990, 26, 1105 CAS.
- SAINT, Version 7.60A, Bruker Analytical X-ray Instruments, Inc., Madison, WI, 2008 Search PubMed.
- A. L. Spek, J. Appl. Crystallogr., 2003, 36, 7 CrossRef CAS.
- (a) P. Kubelka and F. Z. Munk, Tech. Phys., 1931, 12, 593 Search PubMed; (b) J. Tauc, Mater. Res. Bull., 1970, 5, 721 CrossRef CAS.
- S. J. Clark, M. D. Segall, C. J. Pickard, P. J. Hasnip, M. J. Probert, K. Rrfson and M. C. Payne, Z. Kristallogr., 2005, 220, 567 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS.
- J. S. Lin, A. Qteish, M. C. Payne and V. Heine, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 47, 4174 CrossRef.
- A. M. Rappe, K. M. Rabe, E. Kaxiras and J. D. Joannopoulos, Phys. Rev. B: Condens. Matter Mater. Phys., 1990, 41, 1227 CrossRef.
- H. J. Monkhorst and J. D. Pack, Phys. Rev. B: Condens. Matter Mater. Phys., 1976, 13, 5188 CrossRef.
- E. D. Palik, Handbook of Optical Constants of Solids, Academic Press, Orlando, New York, 1985 Search PubMed.
- X. Y. Dong, H. P. Wu, Y. J. Shi, H. W. Yu, Z. H. Yang, B. B. Zhang, Z. H. Chen, Y. Yang, Z. J. Huang, S. L. Pan and Z. X. Zhou, Chem.–Eur. J., 2013, 19, 7338 CrossRef CAS PubMed.
- R. W. Godby, M. Schluter and L. J. Sham, Phys. Rev. B: Condens. Matter Mater. Phys., 1987, 36, 6497 CrossRef CAS.
- F. Wooten, Optical Properties of Solid, Academic, New York, 1972 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Calculated and observed XRD patterns, IR and UV-Vis-NIR diffuse transmittance spectra, substitution of Ge for Si in borates, final refined atomic positions and isotropic thermal parameters, selected bond distances and angles and first-principles calculations for Ba7(BO3)3GeO4X (X = Cl, Br). CCDC 1058752 and 1058753. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra07450a |
| This journal is © The Royal Society of Chemistry 2015 |