Green synthesis of a palladium–polyaniline nanocomposite for green Suzuki–Miyaura coupling reactions†
Abstract
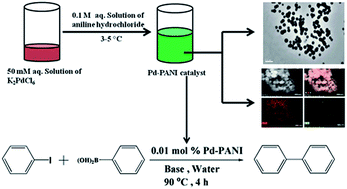
A palladium–polyaniline (Pd–PANI) nanocomposite was successfully synthesized using a one-pot green synthetic procedure in water by the reaction between aniline hydrochloride and potassium hexachloropalladate. Strong interaction between PANI and Pd was clearly evident in the UV-visible and Fourier transform infrared (FTIR) spectrum of the nanocomposite. Powder X-ray diffraction (XRD) patterns revealed the presence of Pd(0) in the nanocomposite with a fcc crystal structure. Field emission scanning electron microscopy (FE-SEM) and transmission electron microscopy (TEM) imaging showed that the Pd–PANI nanocomposite has spherical morphology with an average particle size of 175 ± 42 nm. High resolution TEM imaging and energy dispersive X-ray (EDX) spectroscopy studies revealed that very small nanoparticles of Pd (3.1 ± 0.9 nm) were found dispersed throughout the PANI matrix. X-ray photoelectron spectroscopy (XPS) revealed the presence of Pd(0) in the nanocomposite. The catalytic behavior of the Pd–PANI nanocomposite was studied for Suzuki–Miyaura coupling reactions in the presence of different bases in both organic and aqueous media. The results revealed that the Suzuki–Miyaura reaction proceeds much faster in water than in toluene. The excellent catalytic activity of the nanocomposite resulted in 86 and 91% yield in water and toluene respectively, when potassium carbonate was used as the base.

 Please wait while we load your content...
Please wait while we load your content...