Starch-coated maghemite nanoparticles functionalized by a novel cobalt Schiff base complex catalyzes selective aerobic benzylic C–H oxidation
Maasoumeh Jafarpour*,
Abdolreza Rezaeifard*,
Vahid Yasinzadeh and
Hossein Kargar
Catalysis Research Laboratory, Department of Chemistry, Faculty of Science, University of Birjand, Birjand, 97179-414, Iran. E-mail: mjafarpour@birjand.ac.ir; rrezaeifard@birjand.ac.ir; rrezaeifard@gmail.com; Fax: +98 56 32202515; Tel: +98 56 32202516
First published on 20th April 2015
Abstract
In this work, a novel organosilicon aldehyde was prepared for the first time. It could be used as an appropriate linker by condensation with ethanol amine producing a new bidentate Schiff base ligand. Its cobalt complex was prepared under ultrasonic agitation and characterized by FT-IR and NMR spectra. Attachment to magnetic nanoparticles (γ-Fe2O3, MNP) coated with starch (SMNP) under ultrasonic irradiation produced a magnetically separable Schiff base catalyst. TEM images revealed a uniform spherical shape with average size of 12–15 nm for as-prepared catalyst. The selective benzylic C–H oxidation of a variety of benzylic alcohols as well as alkyl benzenes to the related carbonyl compounds using molecular oxygen in combination with n-hydroxyphthalimide (NHPI) were efficiently enhanced under the catalytic influence of title magnetically separable catalyst. The reactions proceeded smoothly under heterogeneous conditions and catalyst could be recovered and reused efficiently by an external magnetic field. The strong attachment of catalyst to magnetic support was confirmed by FT-IR spectra.
1. Introduction
C–H bonds oxyfunctionalization strategies for the facile generation of compounds with useful molecular architecture remain of high interest to academic and industrial chemists. One major goal is the replacement of stoichiometric procedures, using classical toxic waste-producing oxidants, with catalytic procedures using environmentally benign oxidant. As an alternative, oxygen (or even better air) is among the cheaper and less polluting stoichiometric oxidants, since it produces no waste or water as the sole by-product. Several catalysts and co-catalysts have been applied for the catalytic oxidation of organic substrates.1–4 Indeed, transition metals such as Co(OAc)2,5 CuCl,6 Co(OAc)3,7 and Mn(OAc)3,8 are added in the presence of nonmetallic compounds such as alkyl hydroperoxide,9,10 n-hydroxyphthalimide (NHPI),10–13 azobisisobutyronitril,11 aldehydes12,13 and NO2,14,15 because they are known to catalyze the initiation reaction.4,5,16–23As always in research involving catalysis, attention must also be paid to the vital issue of catalyst integrity, recovery and recycling. Accordingly, heterogeneous catalytic systems are preferred over homogeneous ones due to easier recyclability. However, they usually suffer from low catalytic activity relative to their homogeneous counterparts.
Recently, the use of nanomaterials whose activity is very high under mild conditions extended because of their very large surface area.24–28 The control of chemical reactions by changing the size, dimensionality, chemical composition and morphology of the reaction center and by changing the kinetics using nanopatterning of the reaction centers is the central aim of nanocatalysis.29 However, the small size of nanoparticles make their separation from the reaction solution and recycling difficult, which impedes their use in industrial processes.30 In order to circumvent such recycling problems, magnetic nanoparticles, whose flocculation and dispersion can be controlled reversibly by applying a magnetic field, were extensively employed as a recyclable support matrix in past decade.
As a part of our ongoing research for developing more efficient processes for oxidation of organic compounds,31–43 herein we wish to report the synthesis and catalytic performance of a new cobalt Schiff base complex anchored on magnetic nanoparticles in the aerobic oxidation of benzylic C–H bonds of saturated hydrocarbons and alcohols to the related carbonyl compounds.
The first step of this investigation is the preparation of a bidentate Schiff base ligand by condensation of a new synthesized organosilicon aldehyde with ethanol amine. Its cobalt complex was attached to starch coated magnetic nanoparticles under ultrasonic irradiation producing a magnetically separable nanocatalyst (CoL2@SMNP) (Scheme 1).
The efficiency, selectivity and oxidative stability of title nanocatalyst in the heterogeneous aerobic oxidation of alkyl benzenes and benzyl alcohols in the presence of n-hydroxyphthalimide (NHPI) as radical generator were evaluated which provide its effective reusability and removing by-products (Scheme 2).
2. Experimental
2.1. General remarks
All chemicals were purchased from Merck and Fluka Chemical Companies. Powder X-ray diffraction (XRD) was performed on a Bruker D8-advance X-ray diffractometer with Cu Kα (λ = 1.54178 Å) radiation. The FT-IR spectra were recorded on a NICOLET system. TEM images were obtained by TEM instrumentation 906E (Zeiss, Jena, Germany). Sample for TEM experiment was prepared by dispersing the samples in ethanol, sonicating for 30 min to ensure adequate dispersion of the nanostructures, and evaporating one drop of the solution onto a 200 mesh formbar-coated copper grids.Progresses of the reactions were monitored by TLC using silica-gel SIL G/UV 254 plates and also by GC on a Shimadzu GC-16A instrument using a 25 m CBP1-S25 (0.32 mm ID, 0.5 μm coating) capillary column. NMR spectra were recorded on a Brucker Avance DPX 250 and 400 MHz instruments.
2.2. The preparation of organosilicon aldehyde
To a solution of (3-chloropropyl)trimethoxysilan (0.74 ml, 4 mmol) in DMSO (0.35 ml, 5 mmol) was added NaHCO3 (0.67 g, 8 mmol) and refluxed at 155 °C for 2 h. Then, water (50 ml) was added to the reaction mixture and the product extracted with Et2O (2 × 50 ml). The organic layer was separated and dried over anhydrous CaCl2 and filtered. Evaporation of the solvent afforded the desired product (yellow oil, 70% isolated yield). Structural assignments of the product is based on their FT-IR, 1H NMR (Section 3.1).2.3. The preparation of Schiff base ligand
To a solution of (3-oxopropyl)trimethoxysilan (0.8 ml, 5 mmol) in ethanol (10 ml) was gradually added a solution of ethanol amine (0.3 ml, 5 mmol) in ethanol (10 ml) at 60 °C under ultrasonic agitation and remained under the same conditions for 60 min. The product precipitated after 24 hours at room temperature, which was filtered and washed with ethanol and dried in desiccator (85% isolated yield). Structural assignments of the product is based on their FTIR, 1H NMR (Section 3.1).2.4. The preparation of cobalt Schiff base complex
A solution of cobalt acetate (0.44 g, 2.5 mmol) in ethanol (10 ml) was added to a solution of Schiff base ligand (5 mmol, 0.25 g) in 10 ml ethanol at 60 °C under ultrasonic agitation and the remained under the same conditions for 3 hours. The cobalt Schiff base complex precipitated after cooling the reaction mixture, which was filtered and washed with cold ethanol followed by drying at 100 °C under vacuum (92%). Structural assignments of the product is based on their FTIR, 1H NMR (Section 3.1).2.5. The preparation of γ-Fe2O3
γ-Fe2O3 MNPs were synthesized by a reported chemical co-precipitation technique of ferric and ferrous ions in alkali solution with minor modifications.44FeCl2·4H2O (1.99 g) and FeCl3·6H2O (3.25 g) were dissolved in deionized water (30 ml) under Ar atmosphere at room temperature. A NH4OH solution (0.6 M, 200 ml) was then added drop wise (drop rate = 1 ml min−1) to the stirring mixture at room temperature to reach the reaction pH to 11. The resulting black dispersion was continuously stirred for 1 h at room temperature and then heated to reflux for 1 h to yield a brown dispersion. The magnetic nanoparticles were then separate by an external magnet and washed with deionized water until it was neutralized. The as-synthesized sample was heated at 2 °C min−1 up to 250 °C and then kept in the furnace for 3 h to give a reddish-brown powder.
2.6. The preparation of starch coated γ-Fe2O3@(SMNP)
To 1.0 g dispersed γ-Fe2O3 particles in 15 ml bidistilled water was gradually added a hot aqueous solution of starch under ultrasonic agitation and was kept for an additional 15 min. After stirring at room temperature for 8 h, the starch coated maghemite nanoparticles (SMNP) was separated by an external magnetic field, washed with water and dried.2.7. The immobilization of CoL2 on the SMNP (CoL2@SMNP)
To 1.0 g of SMNP in 30 ml anhydrous toluene was gradually added [CoL2] (2 mmol) (a period of 10 min) under ultrasonic agitation at room temperature and the as-obtained mixture was refluxed for 14 h. The final sample (CoL2@SMNP) was separated by an external magnetic field, washed three times with ethanol and dried under vacuum (Scheme 1).2.8. General procedure for aerobic oxidation of benzyl alcohols
To a mixture of benzyl alcohols (1 mmol) and nanocatalyst (0.5 mol%, 0.03 g) in CH3CN (3 ml) was added NHPI (10 mol%, 0.016 g) and the reaction mixture was stirred at 70 °C under a continuous stream of O2 (5–7 ml min−1) for the required time. The reaction progress was monitored by GC, and the yields of the products were determined by GC and NMR analysis.2.9. General procedure for aerobic oxidation of alkyl benzenes
To a mixture of alkyl benzene (1 mmol) and nanocatalyst (0.5 mol%, 0.03 g) in CH3CN (3 ml) was added NHPI (15 mol%, 0.025 g) and the reaction mixture was stirred at 70 °C under a continuous stream of O2 (5–7 ml min−1) for the required time. The reaction progress was monitored by GC, and the yields of the products were determined by GC and NMR analysis.3. Results and discussion
3.1. Synthesis and characterization of catalyst
At the first step of this work, a new organosilicon aldehyde was prepared by oxidation of (3-chloropropyl)trimethoxysilan in the presence of DMSO and NaHCO3.45 The presence of characteristic carbonyl band at 1742 cm−1 in FT-IR demonstrated well the formation of desired aldehyde that is (3-oxopropyl)trimethoxysilan (Fig. 1a). The bands at 2820 cm−1 can be rationalized to C–H bonds stretching of aldehyde, while the broad peak at 1073 cm−1 assigned to Si–O stretching vibrations (Fig. 1a). The observation of peaks at 3.5 and 9.66 ppm in the 1H NMR spectrum of the (3-oxopropyl)trimethoxysilan (Fig. 2a) assigned to the methoxy and aldehyde proton, respectively. | ||
| Fig. 1 FT-IR spectra of (a) (3-oxopropyl)trimethoxysilan, (b) Schiff base ligand and (c) cobalt Schiff base complex. | ||
 | ||
| Fig. 2 1HNMR spectra of (a) (3-oxopropyl)trimethoxysilan, (b) Schiff base ligand and (c) cobalt Schiff base complex. | ||
Condensation of the synthesized aldehyde with ethanol amine in ethanol produced the Shiff base ligand. The FT-IR spectra (Fig. 1b) represents the characteristic imine band at 1656 cm−1 indicating the successful preparation of the Schiff base ligand.
Then reaction of HL with Co(OAc)2 in ethanol gave the desired complex, CoL2, as a deep purple powder. A lower frequency was observed for imine band in FT-IR spectra of complex indicating of coordination to Co center (1640 cm−1) through azomethine nitrogen. This was further supported by 1H NMR spectra. A sharp singlet at 7.6 ppm in the 1H NMR spectrum of the free Schiff base, (Fig. 2b) assigned to the azomethine proton shifted downfield (7.9 ppm) after complexation (Fig. 2c). Moreover, the absence of a broad singlet at 4.8 ppm in the spectrum of the cobalt complex (Fig. 2c) compared with the uncoordinated ligand indicates the deprotonation and coordination of the hydroxyl group.
At the second step of this work, prepared CoL2 complex was immobilized on the starch coated magnetic nanoparticles (SMNP) using ultrasonic agitation (CoL2@SMNP). The use of a co-precipitation method for preparation of the γ-Fe2O3 (MNP) gave nanoparticles with a diameter of approximately 10 nm.44 It was coated with a dense starch layer (SMNP) followed by loading with CoL2 (CoL2@SMNP).
The XRD pattern of CoL2@SMNP (Fig. 3, right) exhibit characteristic peaks at the same 2θ values as bare γ-Fe2O3 demonstrating that the crystalline structure of iron oxide remains intact after being attached to Co Schiff base complex.
The FT-IR spectra of bare γ-Fe2O3 nanoparticles, SMNP and CoL2@SMNP nanocomplex, have been depicted in Fig. 4. The spectrum of the bare γ-Fe2O3 nanoparticles, (Fig. 4a), exhibits two peaks at 634 and 582 cm−1 and a weak band around 441 cm−1, which are assigned to typical Fe–O vibrations of the maghemite structure.46–50 The spectrum also represents a broad band around 3390 cm−1 corresponding to O–H stretching vibrations of surface hydroxyl groups and adsorbed water. Bending vibration of adsorbed water can be observed at 1600 cm−1.47
In the FT-IR spectra of the SMNP (Fig. 4b), new bands in 1020, 1080 and 1156 cm−1 associated with acetal groups of amylose and amylopectin in starch were appeared. The peaks at 1156 and 1080 cm−1 represent the C–O stretching vibrations in the C–O–H groups, whereas the 1020 cm−1 peak corresponds to the C–O stretching vibration in the C–O–C groups. The peaks at 2922 and 1422 cm−1 related to the C–H stretching vibration of methylene groups. The peaks at 1650 and 3376 cm−1 were assigned to δ(O–H) bending of water and hydrogen bonded hydroxyl groups of amylose and amylopectin of starch, respectively.51–54
The absorption bands at 578 and 630 cm−1 can be assigned to the Fe–O stretching vibration for the γ-Fe2O3 nanoparticles.
Comparison of the FT-IR spectra of CoL2@SMNP nanocomplex Fig. 4c with those of MNP and SMNP confirms the formations of the respective composite according to significant spectral changes. It revealed the presence of major bands at 1643 cm−1 which are attributed to imine band and a strong peak at 1120 cm−1 corresponding to the Si–O band. It can be observed that there is a weak peak at 472 cm−1, which correspond to the Co–N band.
The loading of cobalt complex on the support was determined by atomic absorption spectroscopy, which showed a value of 0.17 mmol g−1 based on Co.
Transmission electron microscopy (TEM) observations clearly revealed spherical CoL2@SMNP nanocomplex with size ranging between 12–15 nm (Fig. 5).
3.2. Catalytic activity of CoL2@SMNP nanocomplex
A systematic examination of the solvent nature was performed in various solvents, such as acetonitrile, acetic acid, dichloroethane, ethyl acetate, toluene, ethanol and water, using 0.5 mol% of CoL2@SMNP in the presence of NHPI (0.1 mmol) at different temperatures (Fig. 6). The best yield and conversion rate were obtained in CH3CN at 70 °C.
The reaction was further optimized with respect to amount of solvent, NHPI and catalyst (Fig. 6).
Inspection of the results in Fig. 6iv revealed that the efficiency of oxidation was dependent on the NHPI amount, so that the reaction did not proceed in the absence of NHPI under any conditions. NHPI has been demonstrated to be a free radical oxidation catalyst.55,56 Therefore, a radical mechanism may be suggested for title oxidation system using O2, similar to previous reports in the presence of cobalt complexes.5,57 This was further supported by retarded oxidation of benzyl alcohol in the presence of radical scavengers such as 2,6-di-tert-butyl-4-methylphenol under the same conditions.
It should be noted that no oxidation product was observed when the title catalyst was replaced by γ-Fe2O3 as well as SMNP. However, the reaction proceeded well in the presence of unsupported CoL2, and the same result was obtained under the same conditions demonstrated well the efficiency of CoL2 preserved after being immobilized on magnetic nanoparticles.
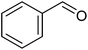
Under the optimized conditions using a continuous stream of O2 at 70 °C, benzyl alcohol converted completely in the presence of 0.5 mol% of CoL2@SMNP within 16 h and 90% of the pertinent aldehyde was secured as the sole product.
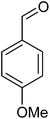
To establish the general applicability of the method, various benzylic alcohols were subjected to the oxidation protocol under the catalytic influence of the title heterogeneous catalyst (Table 1). Different benzylic alcohols oxidized smoothly under oxidation procedure with second benzylic alcohols faster than primary ones. For example, 1-phenyl ethanol and benzyl alcohol were converted to the corresponding carbonyl compounds after 14 and 16 h, respectively (Table 1, entries 1, 11). Our results revealed that, the oxidation performance affected by electronic properties of substrates. Electron-releasing groups on the phenyl rings of alcohols accelerated the reaction (Table 1, entries 2–5), while electron-acceptor ones retarded it (Table 1, entries 8, 9). For example, in the oxidation of 4-nitro- and 4-methoxy benzyl alcohol, 35 and 72% yields of the pertinent aldehydes were obtained respectively at the same time (16 h).
| Entry | Alcohol | Productb | Yieldc % (isolated yield %) |
|---|---|---|---|
a Reaction conditions: 1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 molar ratio for alcohol 5 molar ratio for alcohol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NHPI NHPI![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) catalyst in CH3CN (3 ml) under continues stream of O2 (5–7 ml min−1) at 70 °C.b The products were identified by 1H NMR spectra in comparison with authentic samples.58 The selectivity of products were >99% based on GC analysis.c GC yield after 16 h except for entry 11 which has been run for 14 h. catalyst in CH3CN (3 ml) under continues stream of O2 (5–7 ml min−1) at 70 °C.b The products were identified by 1H NMR spectra in comparison with authentic samples.58 The selectivity of products were >99% based on GC analysis.c GC yield after 16 h except for entry 11 which has been run for 14 h. |
|||
| 1 |  |
 |
97 (90) |
| 2 |  |
 |
72 (65) |
| 3 |  |
 |
72 (65) |
| 4 |  |
 |
57 (49) |
| 5 |  |
 |
77 (70) |
| 6 |  |
 |
44 |
| 7 |  |
 |
60 (54) |
| 8 |  |
 |
35 |
| 9 |  |
 |
44 |
| 10 |  |
 |
52 |
| 11 |  |
 |
100 (92)c |
| 12 |  |
 |
65 (59) |
| 13 |  |
 |
67 (60) |
| 14 |  |
 |
55 (48) |
The chemoselectivity of the procedure was prominent. While, the primary benzylic alcohol containing sulfide group, oxidized to the corresponding aldehyde (52% yield) under the influence of title catalytic system, sulfide group remained completely intact (Table 1, entry 10). It should be noted that no trace of ester and benzoic acid was observed resulting from over oxidation of secondary and primary alcohols, respectively. The catalytic system didn't show any reactivity toward aliphatic alcohols.
The desired yields and selectivities of the carbonyl compounds obtained in the oxidation of benzyl alcohols prompted us to apply this catalytic system for oxidation of alkyl benzenes. Control experiments revealed that the same reaction conditions used for alcohol oxidation can be applicable successfully for oxidation of alkyl benzenes albeit with more amount of NHPI (15 mol%). The complete oxidation of ethyl benzene took 9 h in acetonitrile at 70 °C and acetophenone was obtained quantitatively contaminated with a small percentage of the pertinent alcohol (<3%).
The good/high yields (65–97%) and high selectivity (80–97%) of ketones were achieved in the oxidation of other alkyl benzenes (Table 2, entries 1, 2, 4–6). The conversion for electron-deficient 4-nitroethylbenzene (Table 2, entry 3), was 61% and the pertinent acetophenone was achieved in 72% yield. The method possesses novelty regarding the chemoselectivity. 2-Phenyl ethanol oxidized to 2-hydroxy acetophenone while, hydroxyl group was tolerated in the reaction (Table 2, entry 7).
| Entry | Alkyl benzene | Productb | Yieldc % | Time (h) | Ketone selectivityd % |
|---|---|---|---|---|---|
a Reaction conditions: 1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 150 150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 molar ratio for alcohol 5 molar ratio for alcohol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NHPI NHPI![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) catalyst in CH3CN (3 ml) under O2 (7–10 ml min−1) at 70 °C.b The products were identified by 1H NMR spectroscopy58 or by comparison with the retention times of authentic samples in GC analysis.c GC yield.bd The remainders are the related alcohols.e 2-Adamantanol (7%) and 2-adamantanon (13%) were also produced alongside 1-adamantanol as main product. catalyst in CH3CN (3 ml) under O2 (7–10 ml min−1) at 70 °C.b The products were identified by 1H NMR spectroscopy58 or by comparison with the retention times of authentic samples in GC analysis.c GC yield.bd The remainders are the related alcohols.e 2-Adamantanol (7%) and 2-adamantanon (13%) were also produced alongside 1-adamantanol as main product. |
|||||
| 1 | 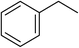 |
 |
97 | 9 | 97 |
| 2 |  |
 |
85 | 8 | 90 |
| 3 |  |
 |
44 | 11 | 72 |
| 4 |  |
 |
76 | 9 | 90 |
| 5 |  |
 |
75 | 9 | 80 |
| 6 |  |
 |
65 | 9 | 90 |
| 7 |  |
 |
77 | 9 | 100 |
| 8 |  |
 |
87 | 9 | —e |
The oxidation of adamantane was efficiently promoted by title catalytic aerobic system. The major product was a tertiary CH bond oxygenated product of 1-adamantanol, and secondary CH bond oxygenated products were formed sparsely.
This was further supported by evaluation of the recovery potential of the nanocatalysts in the oxidation of both benzyl alcohols and alkyl benzenes under the conditions mentioned in Tables 1 and 2.
Recovery of CoL2@SMNP catalyst was easy and efficient.
When the magnetic stirring was stopped, the catalyst absorbed on to the magnetic stirring bar. The catalyst was recovered by decantation of the reaction mixture in the presence of an external magnet. It was then washed with ethylacetate or ethanol as safe solvents, dried under vacuum, and used directly for the next round of reaction without further purification. The ease of recovery, combined with the intrinsic stability of the starch-protected γ-Fe2O3 nanoparticle component, allows the catalyst to be recovered efficiently over at least fifth times in the oxidation of benzyl alcohol under mentioned conditions in this study (Fig. 7).
The FT-IR spectra (Fig. 7) of the used catalyst demonstrated that the structure of the catalyst preserved after recovery.
4. Conclusions
In this research, a new organosilicon aldehyde was prepared by oxidation of (3-chloropropyl)trimethoxysilan that it can be used as a new linker for designing of new heterogeneous supported catalysts. It was condensed with ethanol amine to produce a new Schiff base ligand followed by complexation with Co(OAc)2. The CoL2@SMNP nanocatalyst (12–15 nm) was prepared by immobilizing of cobalt Schiff base complex on the starch coated γ-Fe2O3 nanoparticles.The title nanocatalyst catalyzed heterogeneous aerobic oxidation of alkyl benzenes and benzyl alcohols using NHPI as radical generator. The efficiency, selectivity and oxidative stability of catalyst well documented. Selected processes successfully provided target products in good/excellent yields by using a low catalyst loading of 0.5 mol%, while, its initial activity was preserved after several recoveries regardless of the type of selected reaction. Thus, our method is cost effective which enables the industrially important reactions to be carried out efficiently under aerobic and practically attainable conditions.
Acknowledgements
Support for this work by Research Council of University of Birjand is highly appreciated.References
- V. D. Makawanna, L. J. Garces, J. Liu, J. Cai, Y. C. Son and S. L. Suib, Catal. Today, 2003, 85, 225–233 CrossRef.
- Y. S. Ding, X. F. Chen, S. Sithambaram, S. Gomez, R. Kumar, V. M. B. Crisostomo, S. L. Suib and M. Aindow, Chem. Mater., 2005, 17, 5382–5389 CrossRef CAS.
- A. K. Vannucci, Z. Chen, J. J. Concepcion and T. J. Meyer, ACS Catal., 2012, 2, 716–719 CrossRef CAS.
- Y. Ishii, K. Nakayama, M. Sakaguachi, S. Iwahama and T. Nishiyama, J. Org. Chem., 1995, 60, 3934–3935 CrossRef CAS.
- Y. Yoshino, Y. Hayashi, T. Iwahama, S. Sakaguchi and Y. Ishii, J. Org. Chem., 1997, 62, 6810–6813 CrossRef CAS.
- M. Nechab, C. Einhorn and J. Einhorn, Chem. Commun., 2004, 1500–1501 RSC.
- N. Sawatari, S. Sakaguchi and Y. Ishii, Tetrahedron Lett., 2003, 44, 2053 CrossRef CAS.
- R. Amorati, M. Lucarini, V. Mugnaini and G. F. Pedulli, J. Org. Chem., 2003, 68, 1747–1754 CrossRef CAS PubMed.
- I. W. C. E. Arends, M. Sasidharan, A. Kuhnle, M. Duda, C. Jost and R. A. Sheldon, Tetrahedron, 2002, 58, 9055–9061 CrossRef CAS.
- Y. Aoki, S. Sakaguchi and Y. Ishii, Adv. Synth. Catal., 2004, 346, 199–202 CrossRef CAS PubMed.
- C. Einhorn, J. Einhorn, C. Marcadel and J.-L. Pierre, Chem. Commun., 1994, 447–448 Search PubMed.
- S. Tsujimoto, S. Sakaguchi and Y. Ishii, Tetrahedron Lett., 2003, 44, 5601–5604 CrossRef CAS.
- M. Eikawa, S. Sakaguchi and Y. Ishii, J. Org. Chem., 1999, 64, 4674–4685 CrossRef PubMed.
- Y. Nisshawaki, S. Sakaguchi and Y. Ishii, J. Org. Chem., 2002, 67, 5663–5668 CrossRef PubMed.
- T. Iwahama, S. Sakaguchi, Y. Nishiyama and Y. Nishayama, J. Org. Chem., 1996, 61, 4520–4526 CrossRef PubMed.
- Y. Ishii, T. Iwahama and S. Sakaguchi, Tetrahedron Lett., 1996, 37, 4993–4996 CrossRef CAS.
- Y. Ishii, J. Mol. Catal. A: Chem., 1997, 117, 123–137 CrossRef CAS.
- S. Kato, T. Iwashama, S. Sakaguchi and Y. Ishii, J. Org. Chem., 1998, 63, 22 CrossRef.
- S. Sakaguchi, T. Takase, T. Iwahama and Y. Ishii, Chem. Commun., 1998, 2037–2038 RSC.
- T. Iwahama, S. Sakaguchi and Y. Ishii, Tetrahedron Lett., 1998, 39, 9059–9062 CrossRef CAS.
- T. Iwahama, S. Sakaguchi and Y. Ishii, Chem. Commun., 2000, 7, 613–614 RSC.
- Y. Ishii, Catal. Today, 2000, 117, 105–113 CrossRef PubMed.
- Y. Ishii, S. Sakaguchi and T. Iwahama, Adv. Synth. Catal., 2001, 343, 939–941 CrossRef.
- M. B. Gawande, P. S. Branco and R. S. Varma, Chem. Soc. Rev., 2013, 42, 3371–3393 RSC.
- R. G. Chaudhuri and S. Paria, Chem. Rev., 2012, 112, 2373–2433 CrossRef PubMed.
- L. Guerrini and D. Graham, Chem. Soc. Rev., 2012, 41, 7085–7107 RSC.
- L. H. Reddy, J. L. Arias, J. Nicolas and P. Couvreur, Chem. Rev., 2012, 112, 5818–5878 CrossRef CAS PubMed.
- V. Polshettiwar, R. Luque, A. Fihri, H. Zhu, M. Bouhrara and J.-M. Basset, Chem. Rev., 2011, 111, 3036–3075 CrossRef CAS PubMed.
- D. Astruc, Nanoparticles and Catalysis, Wiley-VCH Verlag GmbH & Co. KGaA, 2008, pp. 1–48 Search PubMed.
- F. Raimondi, G. G. Scherer, R. Kotz and A. Wokaun, Angew. Chem., Int. Ed., 2005, 117, 2228–2248 CrossRef PubMed.
- A. Rezaeifard, R. Haddad, M. Jafarpour and M. Hakimi, J. Am. Chem. Soc., 2013, 135, 10036–10039 CrossRef CAS PubMed.
- A. Rezaeifard, P. Farshid, M. Jafarpour and G. Kardan Moghaddam, RSC Adv., 2014, 4, 9189–9196 RSC.
- A. Rezaeifard and M. Jafarpour, Catal. Sci. Technol., 2014, 4, 1960–1969 CAS.
- M. Jafarpour, M. Ghahramaninezhad and A. Rezaeifard, RSC Adv., 2014, 4, 1601–1608 RSC.
- M. Jafarpour, A. Rezaeifard, M. Ghahramaninezhad and F. Feizpour, Green Chem., 2015, 17, 442 RSC.
- M. Jafarpour, M. Ghahramaninezhad and A. Rezaeifard, New J. Chem., 2014, 38, 2917–2926 RSC.
- A. Rezaeifard, V. Soltani and M. Jafarpour, Eur. J. Inorg. Chem., 2013, 2657–2664 CrossRef CAS PubMed.
- A. Rezaeifard, M. Jafarpour, A. Naeimi and M. Salimi, Inorg. Chem. Commun., 2012, 15, 230–234 CrossRef CAS PubMed.
- A. Rezaeifard, M. Jafarpour, A. Naeimi and K. Mohammadi, J. Mol. Catal. A: Chem., 2012, 357, 141–147 CrossRef CAS PubMed.
- A. Rezaeifard, M. Jafarpour, P. Farshid and A. Naeimi, Eur. J. Inorg. Chem., 2012, 5515–5524 CrossRef CAS PubMed.
- A. Rezaeifard, M. Jafarpour, A. Naeimi and R. Haddad, Green Chem., 2012, 14, 3386–3394 RSC.
- A. Rezaeifard, M. Jafarpour, A. Naeimi and S. Kaafi, Catal. Commun., 2011, 12, 761–765 CrossRef CAS PubMed.
- A. Rezaeifard, M. Jafarpour and A. Naeimi, Catal. Commun., 2011, 16, 240–244 CrossRef CAS PubMed.
- (a) R. Massart, E. Dubois, V. Cabuil and E. Hasmonay, J. Magn. Magn. Mater., 1995, 149, 1–5 CrossRef CAS; (b) B. Z. Tang, Y. Geng, J. W. Y. Lam, B. Li, X. Jing, X. Wang, F. Wang, A. B. Pakhomov and X. X. Zhang, Chem. Mater., 1999, 11, 1581–1589 CrossRef CAS.
- G. Grivani, S. Tangestaninejad and A. Halili, Inorg. Chem. Commun., 2007, 10, 914–917 CrossRef CAS PubMed.
- R. M. Cornell and U. Schwertmann, The Iron Oxides: Structure, Properties, Reactions, Occurrence and Uses, VCH, Weinheim, 1996 Search PubMed.
- T. Belin, N. Guigue-Millot, T. Caillot, D. Aymes and J. C. Niepce, J. Solid State Chem., 2002, 163, 459 CrossRef CAS.
- R. de Palma, J. Trekker, S. Peeters, M. J. Van Bael, K. Bonroy, R. Wirix-Speetjens, G. Reekmans, W. Laureyn, G. Borghs and G. Maes, J. Nanosci. Nanotechnol., 2007, 7, 4626 CAS.
- M. P. Morales, S. Veintemillas-Verdaguer, M. I. Montero, C. J. Serna, A. Roig, L. Casas, B. Martínez and F. Sandiumenge, Chem. Mater., 1999, 11, 3058 CrossRef CAS.
- P. Tartaj, M. P. Morales, S. Veintemillas-Verdaguer, T. Gonz'alez-Carreno and C. J. Serna, J. Phys. D: Appl. Phys., 2003, 36, R182 CrossRef CAS.
- R. Kizil, J. Irudayaraj and K. Seetharaman, J. Agric. Food Chem., 2002, 50, 3912–3918 CrossRef CAS PubMed.
- M. Huang, H. Wang and J. Yu, Polym. Compos., 2006, 309–314 CrossRef PubMed.
- S. M. Goheen and R. P. Wool, J. Appl. Polym. Sci., 1991, 42, 2691 CrossRef CAS PubMed.
- J. F. Mano, D. Koniarova and R. L. Reis, J. Mater. Sci.: Mater. Med., 2003, 14, 127 CrossRef CAS.
- Y. Ishii, K. Nakayama, M. Takeno, S. Sakaguchi, T. Iwahama and Y. Nishiyama, J. Org. Chem., 1995, 60, 3934–3935 CrossRef CAS.
- Y. Ishii, S. Sakaguchi and T. Iwahama, Adv. Synth. Catal., 2001, 343, 393–427 CrossRef CAS.
- (a) C. Parmeggiani and F. Cardona, Green Chem., 2012, 14, 547–564 RSC; (b) Y. Ishii and S. Sakaguchi, Catal. Today, 2006, 117, 105–113 CrossRef CAS PubMed; (c) H. Ma, J. Xu, Q. Zhang, H. Miao and W. Wu, Catal. Commun., 2007, 8, 27–30 CrossRef CAS PubMed; (d) D. Habibi, A. R. Faraji, M. Arshadib, S. Heydari and A. Gil, Appl. Catal., A, 2013, 466, 282–292 CrossRef CAS PubMed; (e) T. Iwahama, S. Sakaguchi, Y. Nishiyama and Y. Ishii, Tetrahedron Lett., 1995, 36, 6923 CrossRef CAS; (f) T. Iwahama, Y. Yoshino, T. Keitoku, S. Sakaguchi and Y. Ishii, J. Org. Chem., 2000, 65, 6502 CrossRef CAS; (g) S. J. S. Kalra, T. Punniyamurthy and J. Iqbal, Tetrahedron Lett., 1994, 35, 4847 CrossRef CAS; (h) A. K. Mandal and J. Iqbal, Tetrahedron, 1997, 53, 7641 CrossRef CAS; (i) V. B. Sharma, S. L. Jain and B. Sain, J. Mol. Catal. A: Chem., 2004, 212, 55 CrossRef CAS PubMed.
- (a) S. K. Hanson, R. Wu and L. A. “Pete” Silks, Org. Lett., 2011, 13, 1908–1911 CrossRef CAS PubMed; (b) B. Landers, C. Berini, C. Wang and O. Navarro, J. Org. Chem., 2011, 76, 1390–1397 CrossRef CAS PubMed; (c) M. B. Gawande, A. Rathi, I. D. Nogueira, C. A. A. Ghumman, N. Bundaleski, O. M. N. D. Teodoro and P. S. Branco, ChemPlusChem, 2012, 77, 865–871 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |







