Dilute acid pretreatment of rice straw, structural characterization and optimization of enzymatic hydrolysis conditions by response surface methodology†
Siddheshwar Dnyandev Kshirsagarab,
Pankajkumar Ramdas Waghmareb,
Prakash Chandrakant Lonic,
Sushama Anandrao Patila and
Sanjay Prabhu Govindwar*b
aDepartment of Biotechnology, Shivaji University, Kolhapur-416004, India
bDepartment of Biochemistry, Shivaji University, Kolhapur-416004, India. E-mail: spg_biochem@unishivaji.ac.in; spgovindwar@rediffmail.com; Fax: +91-231-2691533; Tel: +91-231-2609152
cDepartment of Environmental Biotechnology, Shivaji University, Kolhapur-416004, India
First published on 14th May 2015
Abstract
Efficient conversion of fermentable sugars from cheap lignocellulosic biomass is a current need in viable ethanol production technology. In the present study, agricultural waste biomass such as rice straw was pretreated by using 0.5% sulfuric acid for 60 min at 121 °C in an autoclave. A statistical experimental design like central composite design (CCD) was used for optimization of the enzymatic hydrolysis conditions to achieve a significant reducing sugar yield using commercial cellulase. The optimal conditions for acid pretreated rice straw were found to be 40 FPU g−1 enzymes loading, 17.50% biomass loading at 50 °C for 72 h. The reducing sugar yield was 0.359 g g−1, achieved at the optimized conditions. Experimental results under optimum conditions fit well with CCD model predictions. The structural and morphological changes in native and dilute acid treated rice straw substrate were evaluated by FTIR, XRD and SEM analysis. The XRD pattern of biomass revealed an increase in the crystallite size and crystallinity index of pretreated biomass. Scanning electron micrography reported surface porosity and a distorted structure due to pretreatment. HPTLC analysis of sugars like glucose and xylose in hydrolysate produced after enzymatic hydrolysis was determined.
1. Introduction
The global energy consumption has tremendously increased which has caused a shortage of crude oil reservoirs. Thus, research interest has been increased towards the development of alternative clean energy sources. Plants fix atmospheric carbon dioxide and produce complex carbohydrates by photosynthesis. Thus plants are a renewable energy source, and abundant lignocellulosic biomass has significant potential for biofuel production. Biofuel production by utilizing lignocellulosic biomass is a clean option as compared to nonrenewable energy sources like fossil fuels. Ultimately, it will help to reduce the dependency on fossil fuels for energy sources as well as greenhouse gas emission. At present, the production of second generation biofuels is desirable for control of the crisis related to the environment and society.1Lignocellulose is mainly composed of three major polymers like cellulose, hemicellulose, and lignin. Among which, cellulose and hemicellulose are packed densely in the lignocellulosic biomass while layer of lignin offer the resistance against enzymatic hydrolysis. Hence the pretreatment process is required for disruption of lignin layer and for easier accessibility of hydrolytic enzymes to cellulose and hemicellulose.2 However lignocellulosic biomass can be converted into simpler and more biodegradable form by the pretreatment protocol like enzymatic hydrolysis of cellulose. The composition of lignocellulosic biomass depends upon various factors i.e. genetic variability, climate, soil type, environmental influences, etc.3,4
Rice is one of the most important staple food crops grown in major farmland of India. Agricultural waste biomass such as rice straw is produced after post-cultivation of rice crop. Conversion of rice straw biomass to biofuels or other chemicals give rise to proper and efficient utilization of biomass.5,6 To achieve best yield of fermentable sugar, pretreatment is strictly required in case of lignocellulosic waste biomass. There are various processes reported for the conversion of lignocellulosic biomass into biofuels production i.e. pretreatments, saccharification and fermentation.7 Pretreatments are required for increasing accessibility of surface area of cellulose for reducing sugar production by removing components such as hemicelluloses and lignin from the complex structure. Due to chemical pretreatment, cellulose can be easily digested in enzymatic saccharification procedure and thus increasing yield of fermentable sugars. Enzymatic hydrolysis is an ecofriendly strategy as well as energy efficient process. Comparatively less amount of energy is required for the production of reducing sugars from lignocellulosic biomass. Various efficient pretreatments are required to lignocellulosic biomass before enzymatic hydrolysis for the considerable yield of fermentable sugars.8
The fermentable sugars produced using enzymatic hydrolysis from dilute acid pretreated biomass considered as the most promising for bioethanol production.9 Dilute acid pretreatment helps in dissolution of cellulose and hydrolysis of hemicellulose by which sugar recovery increases in liquid fraction resulting in more availability of cellulose for enzymatic hydrolysis.10–12 In enzymatic saccharification procedure biomass loading, enzyme loading, surfactant concentration and incubation time are the key factors that influences the efficiency of process. Optimization of enzymatic saccharification by conventional technique is time consuming as effect of single component is observed at a time. Response surface methodology (RSM) is a statistical method mainly used for modeling and optimization of the process. RSM gives correct scientific information about the optimum conditions. There are many reports of RSM used for enzymatic saccharification of wheat straw,13,14 switch grass,15 prairie cord grass,16 rice straw17,18 and sugarcane tops.19
In the present work, statistical method such as response surface methodology is used for the optimization of conditions for the production of reducing sugars using enzymatic hydrolysis of dilute acid pretreated rice straw. Central composite design is used to optimize various parameters of enzymatic hydrolysis such as biomass loading, incubation time, enzyme loading and surfactant loading for maximum yield of reducing sugars. The change in functional groups, cellulose crystallinity and structure of rice straw after dilute acid pretreatment was also investigated by using FTIR, XRD and SEM. This study reveals the change in the hydrolysis efficiency of lignocellulosic biomass after dilute acid pretreatment for the generation of liquid biofuel like bioethanol.
2. Experimental
2.1. Raw materials and chemicals
Rice straw a lignocellulosic waste was collected from local farmer, Kolhapur district, Maharashtra, India. The lignocellulosic waste was dried, milled using pulverizer, grinded to particle size <1 mm and stored at 37 °C. The commercial enzymes cellulase (Celluclast® 1.5 L), hemicellulase and β-glucosidase were obtained from Sigma Aldrich, USA. Tween-20 and other all chemicals used were analytical grade purchased from HI-Media (Mumbai).2.2. Biomass pretreatment
Pretreatment with dilute sulfuric acid of rice straw was carried out using 250 mL screw cap bottle containing 10% (w/w) biomass loaded with 0.5% (w/v) H2SO4 and autoclaved (at 120 °C and 15 lb pressure for 60 min). Obtained solid biomass was washed with hot water until it attends pH 7.0. The biomass was dried and used for enzymatic hydrolysis process.2.3. Compositional analysis of rice straw feedstock
The cellulose, hemicellulose and lignin composition in native and pretreated rice straw were analyzed.20 For determination of hemicelluloses, 1 g biomass was treated with 0.5 M NaOH solution (150 mL) and kept at 80 °C for 3.5 h. Further, it was washed to remove Na+ ions (pH 7) and dried to a constant weight. Weight difference between before and after treatment of the sample was considered as the hemicellulose content. For acid soluble lignin, 0.5 g of hemicelluloses-free biomass was treated with 15 mL of 98% H2SO4 and incubated for 2 h at 30 °C. The sample mixture was diluted to 4% H2SO4 by de-ionized water, further autoclaved at 121 °C for 1 h. Filtrate aliquots of diluted sample were measured at the absorbance of 205 nm. Sulfate ions were removed by titration with 10% barium chloride from the remaining biomass and then dried to constant weight. This is considered as lignin content. The difference between total content and hemicelluloses plus lignin content corresponds to the cellulose content.212.4. Enzymatic hydrolysis
Enzymatic saccharification of pretreated rice straw was performed in 100 mL reagent bottle with screw cap by incubating 5 g of biomass in 50 mM citrate buffer (pH 4.8). Hydrolysis was performed using a commercial cellulase (Celluclast 1.5 L), hemicellulase from Aspergillus niger and β-glucosidase from almonds (Sigma Aldrich). The different combination ratio of enzymes were checked for enzymatic hydrolysis (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 1
0, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5
2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 0
2 and 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and the enzyme combination ratio of (1
1), and the enzyme combination ratio of (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5
2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) were used for final enzymatic hydrolysis. The sample was incubated at 50 °C for 48 h in shaking incubator at 150 rpm. The reducing sugar produced in the hydrolysate was determined by 3,5-dinitrosalicylic acid (DNS) method.22
2) were used for final enzymatic hydrolysis. The sample was incubated at 50 °C for 48 h in shaking incubator at 150 rpm. The reducing sugar produced in the hydrolysate was determined by 3,5-dinitrosalicylic acid (DNS) method.22
2.5. Optimization of enzymatic hydrolysis using response surface methodology and central composite design
Central composite design (CCD) was employed to study the effect of independent variables on the response and mutual interactions among them. The variables selected were biomass loading, incubation time, enzyme loading, and surfactant loading. In this study four variables effect at three different levels and a total of 30 experimental runs were obtained. The software Design expert 9 (Stat-Ease, Inc., USA) was used for experimental design, data analysis and quadratic model building. The experimental setup of RSM is shown in Table 1.| Variable | Symbol coded | Actual and coded values | ||||
|---|---|---|---|---|---|---|
| −2 | −1 | 0 | 1 | 2 | ||
| Biomass loading (% w/w) variable | A | 10 | 12.5 | 15 | 17.5 | 20 |
| Incubation time (h) | B | 0 | 24 | 48 | 72 | 96 |
| Enzyme loading (FPU g−1) | C | 10 | 20 | 30 | 40 | 50 |
| Surfactant concentration (% w/w) | D | 0 | 0.050 | 0.125 | 0.200 | 0.275 |
2.6. HPTLC analysis for determination of sugars
The reducing sugars produced after enzymatic hydrolysis was determined by HPTLC technique. In this analysis, silica gel 60 F254 (Merck) HPTLC plate was used. Silica gel plate activation was done by using 0.02 M sodium acetate. After the plate activation, 10 μL of hydrolyzed samples and sugar standards of glucose, xylose and cellobiose were applied on plate by micro syringe using automatic nitrogen gas sample applicator (LinomatV, CAMAG, Switzerland). 8.0 mm bands size with track distance of 14.5 mm, 8.0 mm distance from the lower edge, and 20 mm distance from both edges were used. For chromatographic separation of sugars, mobile phase used was butanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol
ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (5
water (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v). The development of plate was carried out in the twin-trough chamber 10 × 10 cm (CAMAG) up to 85 mm migration distance. For detection of sugars, 0.3% α-naphthol in 5% H2SO4 in methanol solution was used for HPTLC plate.23 Data processing and all instrumentation were performed with the software platform winCATS 1.4.4.6337 (CAMAG).
3 v/v). The development of plate was carried out in the twin-trough chamber 10 × 10 cm (CAMAG) up to 85 mm migration distance. For detection of sugars, 0.3% α-naphthol in 5% H2SO4 in methanol solution was used for HPTLC plate.23 Data processing and all instrumentation were performed with the software platform winCATS 1.4.4.6337 (CAMAG).
2.7. Structural characterization of biomass
| CrI (%) = [(I002−Iam)/I002] × 100 |
The crystallite size (D) was calculated based on the following equation
D = K × λ/β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cosθ cosθ |
D (hkl) is the size of the crystallite (nm), K is the Scherrer constant (0.94), l is X-ray wavelength (for copper, 0.15418 nm). β is the full-width at half-maximum of the reflection hkl, measured at 2θ which is the corresponding Bragg angle.25
The degree of crystallinity was calculated on the basis of area under crystalline peaks and non-crystalline region using following equation:
| Xc = Fc/(Fa + Fc) × 100 |
3. Results and discussion
3.1. Dilute acid pretreatment of rice straw
The lignocellulosic biomass composition of rice straw was investigated before and after pretreatment with dilute acid. Composition of cellulose, hemicellulose and lignin content of native, and dilute acid pretreated biomass were shown in Table 2. The hemicellulose content was 19% in pretreated rice straw and 28% in native rice straw. It may be due to its amorphous nature and can easily hydrolyze during the dilute acid pretreatment. Cellulose content in acid pretreated rice straw was increased (47%) due to dissolution of amorphous materials from the biomass. It also results into increase in cellulose accessibility and maximum fermentable sugar production during enzymatic hydrolysis. The similar results were reported by many research groups.27,28| Rice straw | Cellulose (%) | Hemicellulose (%) | Lignin (%) |
|---|---|---|---|
| Native | 32 | 28 | 17 |
| Dilute acid pretreated | 47 | 19 | 20.5 |
3.2. Optimization of enzymatic hydrolysis
Response surface methodology and central composite design was employed to optimize the parameters of biomass loading, incubation time, enzyme loading, and surfactant concentration for improved reducing sugar yield (Table 4). The reducing sugar (g g−1) and variables interactional relationship were shown in terms of coded values. The response predictions were analyzed by using coded values.| Reducing sugar (g g−1) = 0.26 + 0.025A + 0.034B + 0.027C − 1.218E − 003D − 3.764E − 003AB − 0.014AC + 8.080E − 003AD − 7.742E − 003BC + 1.037E − 003BD + 9.575E − 003CD + 9.897E − 003A2 − 9.666E − 003B2 + 0.011C2 + 7.179E − 003D2 | (1) |
| Rice straw | IR crystallinity ratio | ||
|---|---|---|---|
| A1375/A1512 | A1427/A895 | A1372/A2900 | |
| Native | 1.28 | 2.29 | 0.46 |
| Dilute acid pretreated | 1.19 | 2.18 | 0.47 |
| Run | Biomass loading (% w/w) | Incubation time (h) | Enzyme loading (FPU g−1) | Surfactant concentration (% w/w) | Reducing sugar (g g−1) | |
|---|---|---|---|---|---|---|
| Actual value | Predicted value | |||||
| 1 | 12.5 | 24 | 20 | 0.2 | 0.15 | 0.15 |
| 2 | 15 | 0 | 30 | 0.125 | 0.14 | 0.15 |
| 3 | 15 | 48 | 30 | 0.125 | 0.24 | 0.26 |
| 4 | 17.5 | 24 | 40 | 0.05 | 0.29 | 0.28 |
| 5 | 17.5 | 24 | 20 | 0.05 | 0.26 | 0.26 |
| 6 | 10 | 48 | 30 | 0.125 | 0.24 | 0.25 |
| 7 | 12.5 | 72 | 40 | 0.05 | 0.32 | 0.33 |
| 8 | 17.5 | 24 | 20 | 0.2 | 0.26 | 0.25 |
| 9 | 15 | 48 | 30 | 0.125 | 0.26 | 0.26 |
| 10 | 17.5 | 72 | 20 | 0.2 | 0.33 | 0.33 |
| 11 | 20 | 48 | 30 | 0.125 | 0.35 | 0.35 |
| 12 | 15 | 48 | 30 | 0.125 | 0.27 | 0.26 |
| 13 | 12.5 | 24 | 20 | 0.05 | 0.19 | 0.19 |
| 14 | 12.5 | 72 | 20 | 0.2 | 0.23 | 0.24 |
| 15 | 15 | 48 | 10 | 0.125 | 0.26 | 0.25 |
| 16 | 12.5 | 24 | 40 | 0.2 | 0.27 | 0.27 |
| 17 | 17.5 | 24 | 40 | 0.2 | 0.31 | 0.31 |
| 18 | 15 | 48 | 30 | 0.125 | 0.27 | 0.26 |
| 19 | 15 | 48 | 30 | 0.275 | 0.29 | 0.29 |
| 20 | 12.5 | 72 | 20 | 0.05 | 0.29 | 0.28 |
| 21 | 17.5 | 72 | 20 | 0.05 | 0.32 | 0.33 |
| 22 | 12.5 | 24 | 40 | 0.05 | 0.28 | 0.27 |
| 23 | 15 | 96 | 30 | 0.125 | 0.30 | 0.29 |
| 24 | 15 | 48 | 50 | 0.125 | 0.35 | 0.36 |
| 25 | 15 | 48 | 30 | 0.125 | 0.29 | 0.26 |
| 26 | 15 | 48 | 30 | 0.125 | 0.24 | 0.26 |
| 27 | 17.5 | 72 | 40 | 0.05 | 0.33 | 0.32 |
| 28 | 17.5 | 72 | 40 | 0.2 | 0.36 | 0.36 |
| 29 | 15 | 48 | 30 | −0.025 | 0.28 | 0.29 |
| 30 | 12.5 | 72 | 40 | 0.2 | 0.34 | 0.33 |
Eqn (1) is the predicted values of the reducing sugar (g g−1), where A, B, C, and D are the coded values of biomass loading, incubation time, enzyme loading, and surfactant concentration, respectively. Validation of the statistical results and the model equation were analyzed using analysis of variance (ANOVA) presented in Table 5. The CCD model fits well and significant showing the F value of 24.89 and Prob > F of less than 0.0001.
| Source | SS | df | MS | F Value | p-value Prob > F | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a SS-sum of squares, MS-mean square, df-degree of freedom. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Model | 0.079 | 14 | 2.273 × 104 | 24.89 | <0.0001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Residual | 3.410 × 103 | 15 | 1.789 × 104 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lack of fit | 1.789 × 103 | 10 | 3.240 × 104 | 0.55 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pure error | 1.620 × 103 | 5 | 2.273 × 104 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cor total | 0.083 | 29 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The cellulase enzyme and the cellulosic substrates interaction is a very complicated process as hydrolysis efficiency is affected by the various variables in the process. Enzymatic hydrolysis of biomass loading, incubation time, enzyme concentration and surfactant concentration variables were analyzed at a time. The total 30 experimental runs were completed at different combinations of variables and the observed responses were presented in Table 4. The interactive effects of variables on reducing sugar yield were evaluated by three dimensional surface plots for obtaining maximum response and optimum level.
Fig. 1A represents the mutual interaction between biomass loading and incubation time which affect the reducing sugar concentration (g g−1). The low concentration of biomass loading (12.5%) and minimum incubation time gives lower yield, whereas the higher incubation time (72 h) significantly affects the reducing sugar concentration. The yield increased proportionally with an incubation time and biomass loading. The results demonstrated that, an increase in the hydrolysis time from 24 to 72 h produces statistically higher saccharification yields in case of acid pretreated rice straw. The higher the biomass concentration and higher incubation time gives maximum yield. The highest reducing sugar production was reached at 72 h of hydrolysis. The biomass (17.5%) and enzyme loading concentration (40 FPU g−1) showed maximum reducing sugar production (Fig. 1B).
| Source | Substrate | Reducing sugar (mg g−1-substrate) | Ref. |
|---|---|---|---|
| Celluclast 1.5 L & Novozyme 188 | Populus tormentosa Carr. | 290 | 36 and 37 |
| Celluclast 1.5 L & Novozyme 188 | Olea europaea | 302 | 38 |
| Spezyme CP & Novozyme 188 | Populus tremuloides | 301 | 39 |
| Celluclast 1.5 L & Novozyme 188 | Olive tree | 331 | 40 |
| Celluclast 1.5 L & Cellulase from Trichoderma reesei and xylanase from Trametes versicolor (Sigma Aldrich®) | Yellow poplar | 385 | 41 |
| Water hyacinth biomass | 411 | 42 | |
| Celluclast 1.5 L & Novozyme 188 | Populus nigra | 378 | 43 |
| Commercial cellulase (Zytex India Private limited, India) | Sugarcane tops | 531 | 19 |
| Celluclast 1.5 L, β-glucosidase from almonds and hemicellulase from Aspergillus niger (Sigma Aldrich) | Rice straw | 359 | This study |
The interactive effect of biomass loading and surfactant concentration on reducing sugar yield is shown in Fig. 1C. Surfactants have a positive effect on enzymatic hydrolysis. Addition of surfactants to enzymatic hydrolysis of lignocelluloses increased the conversion of cellulose into soluble sugars.29 Surfactants reduce the unproductive enzyme adsorption to the lignin part of the biomass.28 This is due to hydrophobic interaction of surfactant with lignin which releases nonspecifically bound enzyme. Reducing sugar was increased at the surfactant concentration increases.
The interactional effects of incubation time and enzyme loading on reducing sugar yield were presented in Fig. 1D. Low concentration of enzyme loading and low incubation time gave lower reducing sugar yield. Similarly the moderate level of incubation time and high level of enzyme loading gives higher reducing sugar yield. Both parameters showed mutual interactional effects on each other.
Fig. 1E explains the interaction of incubation time and surfactant concentration on the yield of reducing sugar. Maximum reducing sugar yield were achieved by low surfactant concentration and moderate incubation time. Surfactant concentration has positive interactional effect with incubation time for reducing sugar production.
Effect of enzyme loading and surfactant concentration on the yield of reducing sugar of acid pretreated rice straw is presented in Fig. 1F enzyme loading and surfactant concentration had mutual interactional effects on each other. Higher reducing sugar yield was obtained by increasing enzyme loading concentration at moderate surfactant concentration. Table 6 reveals the enzymatic hydrolysis of various substrates and reducing sugar production using commercial cellulase enzymes.
RSM study reports the optimum conditions for enzymatic hydrolysis of acid pretreated rice straw as biomass loading (17.50% w/w), enzyme loading (40 FPU g−1), surfactant concentration (0.2% w/w) and incubation time (72 h) with reducing sugar yield of 0.359 g g−1. Untreated rice straw after enzymatic hydrolysis produced 0.107 g g−1 of reducing sugar. The results indicated that acid pretreatment and combination of cellulase cocktail ratio improved the hydrolysis yield by 3.36 fold. And using only single enzyme and low biomass loading reducing sugar yield was very low. The biomass loading (4.86% w/w), enzyme loading (29.45 FPU), surfactant concentration (0.13% w/w) and incubation time (47.35 h) with reducing sugar yield of 0.184 g g−1. Untreated rice straw after enzymatic hydrolysis produced 0.089 g g−1 of reducing sugar. The results indicated that acid pretreatment improved the hydrolysis yield by 2.06 fold.
3.3. HPTLC analysis
High performance thin layer chromatography (HPTLC) technique was used for qualitative analysis of reducing sugars produced after enzymatic hydrolysis of dilute acid pretreated rice straw. HPTLC results confirmed the production of glucose and xylose. The profile of reducing sugar produced after enzymatic hydrolysis in hydrolysate was shown in Fig. 5. The sugar standards used were glucose, xylose and cellobiose for the comparison of pretreated rice straw hydrolysate. The results indicated the production of xylose and glucose, where as no cellobiose and galactose were observed. | ||
| Fig. 4 Scanning electron Microscopy (SEM) micrographs of rice straw biomass under 500× and 2000× magnification (A) native (control), (B) dilute acid pretreated. | ||
 | ||
| Fig. 5 HPTLC analysis of enzymatic hydrolysate of dilute acid pretreated rice straw biomass. Standard: Glu-glucose, Xyl-xylose, Cel-cellobiose; samples run number: A-27, B-28, C-29, D-30. | ||
3.4. Surface characterization
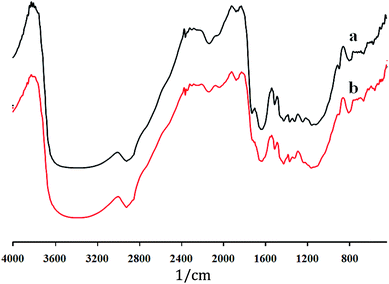
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching of the aromatic ring of lignin.30 1320 and 1430 cm−1 peaks were defined as for the symmetric CH2 bending and wagging,31,32 the peak at 1373 cm−1 assigned for the C–H bending.32 The glycosidic bond of C–O–C stretching at 1160 and 901 cm−1 were earlier reported.31,33 The broad peak at 3300 and 2900 cm−1 defined for the stretching C–H and H–bonded OH groups.32 Fig. 2 demonstrates the structural changes and removal of hemicellulosic components due to acid pretreatment. The 1725 cm−1 peak was absent due to acid hydrolysis of hemicelluloses which is present in native rice straw. Difference in the native and dilute acid pretreated rice straw FTIR spectral pattern suggests the change in the surface structure of the biomass.
C stretching of the aromatic ring of lignin.30 1320 and 1430 cm−1 peaks were defined as for the symmetric CH2 bending and wagging,31,32 the peak at 1373 cm−1 assigned for the C–H bending.32 The glycosidic bond of C–O–C stretching at 1160 and 901 cm−1 were earlier reported.31,33 The broad peak at 3300 and 2900 cm−1 defined for the stretching C–H and H–bonded OH groups.32 Fig. 2 demonstrates the structural changes and removal of hemicellulosic components due to acid pretreatment. The 1725 cm−1 peak was absent due to acid hydrolysis of hemicelluloses which is present in native rice straw. Difference in the native and dilute acid pretreated rice straw FTIR spectral pattern suggests the change in the surface structure of the biomass.IR crystallinity was investigated for native and acid pretreated samples using three IR ratios as A1375/A1512, A1427/A895 and A1372/A2900 of their peak heights, 1375 cm−1 peak for cellulose and the peak at 1512 cm−1 for lignin.30 IR crystallinity ratio of A1375/A1512 in dilute acid pretreated rice straw was lower (1.19) than native rice straw biomass (1.28). Similarly, IR crystallinity ratio of A1427/A895 was lower as compared to native biomass. IR crystallinity ratio of A1372/A2900 was slightly higher in acid pretreated biomass (0.47) than native biomass (0.46) (Table 3), that might be due to amorphous cellulose in acid solution with hemicellulosic components as reported earlier.32
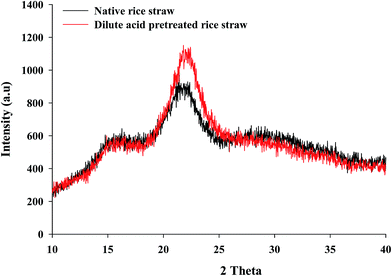
Ionic liquid, steam explosion, lime and dilute acid pretreated sweet sorghum bagasse substrate showed highest crystalline size as compared to untreated sample.35 The dilute acid pretreated rice straw showed higher crystallinity degree (67.2%) when compared to native (59.37%). Increase in degree of crystallinity due to the effect of acid pretreatment was more on amorphous region than the crystalline region. The identical observations are also reported earlier.31
4. Conclusion
The efficiency of enzymatic hydrolysis was found to increase after the dilute acid pretreatment of rice straw. Response surface methodology, the central composite design is found to be useful in identifying the important factors influencing enzymatic conversion of pretreated rice straw carbohydrate. The experimental results revealed interactions of biomass loading and enzyme loading with surfactant concentration. The combinational interactions significantly affect the saccharification efficiency in the pretreated rice straw. Reducing sugar yield had a good correlation with experimental and predicted results. Present study concludes that dilute acid pretreatment of rice straw provides a better option for increased enzymatic hydrolysis efficiency and also act as a good substrate for bioethanol production.Acknowledgements
The author Siddheshwar Dnyandev Kshirsagar would like to acknowledge UGC-BSR (University Grants Commission), New Delhi for providing financial assistance through UGC-BSR-JRF fellowship during this research work. One of the authors Pankajkumar Ramdas Waghmare would like to acknowledge UGC (University Grants Commission), New Delhi for providing financial assistance through UGC-NET-JRF fellowship during this research work.References
- J. Tollefson, Nature, 2008, 451, 880–883 CrossRef CAS PubMed.
- P. R. Waghmare, A. A. Kadam, G. D. Saratale and S. P. Govindwar, Bioresour. Technol., 2014, 168, 136–141 CrossRef CAS PubMed.
- M. Balat, Energy Convers. Manage., 2011, 52, 858–875 CrossRef CAS PubMed.
- R. K. Sharma and D. S. Arora, Biodegradation, 2011, 22, 143–152 CrossRef CAS PubMed.
- S. H. Lee, T. V. Doherty, R. J. Linhardt and J. S. Dordick, Biotechnol. Bioeng., 2009, 102, 1368–1376 CrossRef CAS PubMed.
- M. E. Himmel, S. Y. Ding, D. K. Johnson, W. S. Adney, M. R. Nimlos, J. W. Brady and T. D. Foust, Science, 2007, 315, 804–807 CrossRef CAS PubMed.
- R. Sindhu, M. Kuttiraja, P. Binod, K. U. Janu, R. K. Sukumaran and A. Pandey, Bioresour. Technol., 2011, 102, 109–115 CrossRef PubMed.
- X. Zhao, L. Zhang and D. Liu, Bioresour. Technol., 2008, 99, 3729–3736 CrossRef CAS PubMed.
- A. T. W. M. Hendriks and G. Zeeman, Bioresour. Technol., 2009, 100, 10–18 CrossRef CAS PubMed.
- M. Linde, E. Jakobsson, M. Galbe and G. Zachhi, Biomass Bioenergy, 2008, 32, 326–332 CrossRef CAS PubMed.
- P. Sassner, C. Martensson, M. Galbe and G. Zacchi, Bioresour. Technol., 2008, 99, 137–145 CrossRef CAS PubMed.
- B. Yang and C. E. Wyman, Biofuels, Bioprod. Biorefin., 2008, 2, 26–40 CrossRef CAS PubMed.
- B. Qi, X. Chen, F. Shen, Y. Su and Y. Wan, Ind. Eng. Chem. Res., 2009, 48, 7346–7353 CrossRef CAS.
- M. Marcos, M. T. Garcıa-Cubero, G. Gonzalez-Benito, M. Coca, S. Bolado and S. Lucas, J. Chem. Technol. Biotechnol., 2013, 88, 237–246 CrossRef CAS PubMed.
- C. Karunanithy and K. Muthukumarappan, Ind. Crops Prod., 2011, 33, 188–199 CrossRef CAS PubMed.
- C. Karunanithy and K. Muthukumarappan, Biochem. Eng. J., 2011, 54, 71–82 CrossRef CAS PubMed.
- R. Sindhu, P. Binod, K. U. Janu, R. K. Sukumaran and A. Pandey, World J. Microbiol. Biotechnol., 2012, 28, 473–483 CrossRef CAS PubMed.
- H. Ma, W. W. Liu, X. Chen, Y. J. Wu and Z. L. Yu, Bioresour. Technol., 2009, 100, 1279–1284 CrossRef CAS PubMed.
- R. Sindhu, M. Kuttiraja, P. Binod, R. K. Sukumaran and A. Pandey, Renewable Energy, 2014, 62, 362–368 CrossRef CAS PubMed.
- H. P. Yang, R. Yan, H. P. Chen, C. G. Zheng, D. H. Lee and D. T. Liang, Energy Fuels, 2006, 20, 388–393 CrossRef CAS.
- F. Xin and A. Geng, Appl. Biochem. Biotechnol., 2010, 162, 295–306 CrossRef CAS PubMed.
- G. M. Miller, Anal. Chem., 1959, 31, 426–428 CrossRef CAS.
- M. Narra, G. Dixit, J. Divecha, D. Madamwar and A. R. Shah, Bioresour. Technol., 2012, 121, 355–361 CrossRef CAS PubMed.
- L. Segal, J. J. Creely Jr, A. E. Martin and L. M. Conrad, Text. Res. J., 1959, 29, 786–794 CrossRef CAS PubMed.
- Y. Cao and H. Tan, Enzyme Microb. Technol., 2005, 36, 314–317 CrossRef CAS PubMed.
- K. Karthika, A. B. Arun and P. D. Rekha, Carbohydr. Polym., 2012, 90, 1038–1045 CrossRef CAS PubMed.
- G. D. Saratale, S. D. Kshirsagar, V. T. Sampange, R. G. Saratale, S. E. Oh, S. P. Govindwar and M. K. Oh, Appl. Biochem. Biotechnol., 2014, 174, 2801–2817 CrossRef CAS PubMed.
- X. Gao, R. Kumar, S. Singh, B. A. Simmons, V. Balan, B. E. Dale and C. E. Wyman, Biotechnol. Biofuels, 2014, 7, 71 CrossRef PubMed.
- T. Erikkson, J. Borjesson and F. Tjerneld, Enzyme Microb. Technol., 2002, 31, 353–364 CrossRef.
- F. Lionetto, R. D. Sole, D. Cannoletta, G. Vasapollo and A. Maffezzoli, Materials, 2012, 5, 1910–1922 CrossRef CAS PubMed.
- Y. Cao and H. Tan, J. Mol. Struct., 2004, 705, 189–193 CrossRef CAS PubMed.
- P. Binod, K. Satyanagalakshmi, R. Sindhu, K. U. Janu, R. K. Sukumaran and A. Pandey, Renewable Energy, 2012, 37, 109–116 CrossRef CAS PubMed.
- P. Sannigrahi, S. J. Miller and A. J. Ragauskas, Carbohydr. Res., 2010, 345, 965–970 CrossRef CAS PubMed.
- R. Samuel, Y. Pu, M. Foston and A. J. Rgauskas, Biofuels, 2010, 1(1), 85–90 CrossRef CAS.
- J. Zhang, X. Ma, J. Yu, X. Zhang and T. Tan, Bioresour. Technol., 2011, 102, 4585–4589 CrossRef CAS PubMed.
- K. Wang, H. Y. Yang, F. Xu and R. C. Sun, Bioresour. Technol., 2011, 102, 4524–4529 CrossRef CAS PubMed.
- Y. Wang, M. Radosevich, D. Hayes and N. Labbé, Biotechnol. Bioeng., 2011, 108, 1042–1048 CrossRef CAS PubMed.
- C. Cara, E. Ruiz, I. Ballesteros, M. J. Negro and E. Castro, Process Biochem., 2006, 41, 423–429 CrossRef CAS PubMed.
- J. R. Jensen, J. E. Morinelly, K. R. Gossen, M. J. Brodeur-Campbell and D. R. Shonnard, Bioresour. Technol., 2010, 101, 2317–2325 CrossRef CAS PubMed.
- C. Cara, E. Ruiz, J. M. Oliva, F. Saez and E. Castro, Bioresour. Technol., 2008, 99, 1869–1876 CrossRef CAS PubMed.
- J. McMillan, M. Newman, D. Templeton and A. Mohagheghi, Appl. Biochem. Biotechnol., 1999, 79, 649–665 CrossRef.
- S. Das, A. Bhattacharya, S. Haldar, A. Ganguly, S. Gu, Y. P. Ting and P. K. Chatterjee, Sustainable Mater. Technol., 2015, 3, 17–28 CrossRef PubMed.
- M. Negro, P. Manzanares, I. Ballesteros, J. Oliva, A. Cabanas and M. Ballesteros, Appl. Biochem. Biotechnol., 2003, 105, 87–100 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra04430h |
| This journal is © The Royal Society of Chemistry 2015 |