Concentrated gelatin/alginate composites for fabrication of predesigned scaffolds with a favorable cell response by 3D plotting
Yongxiang Luo*ab,
Anja Lodea,
Ashwini Rahul Akkinenia and
Michael Gelinskya
aCentre for Translational Bone, Joint and Soft Tissue Research, University Hospital Carl Gustav Carus and Faculty of Medicine, Technische Universität Dresden, Germany. E-mail: luoyongxiang@mail.sic.ac.cn; Fax: +86 21 52413122; Tel: +86 21 52412808
bState Key Laboratory of High Performance Ceramics and Superfine Microstructure, Shanghai Institute of Ceramics, Chinese Academy of Sciences, Shanghai 200050, People's Republic of China
First published on 1st May 2015
Abstract
In the present work, gelatin/alginate and gelatin/alginate/hydroxyapatite (HAP) composite scaffolds were fabricated by 3D plotting based on high concentration gelatin/alginate pastes. At temperatures of 37 °C or above, the developed pastes could be easily processed into designed 3D structures; sequential crosslinking with Ca2+ ions (effective for alginate) and the carbodiimide EDC (effective for gelatin) resulted in stable scaffolds. Mechanical testing demonstrated that the plotted composite scaffolds had a significantly higher strength and modulus compared to most reported gelatin scaffolds prepared by conventional methods. Cell experiments with human bone marrow-derived mesenchymal stem cells (hBMSC) revealed that the gelatin/alginate composite scaffolds favor cell adhesion and support proliferation. Furthermore, the cells showed a homogeneous distribution and excellent migration in the inner regions of the plotted composite scaffolds over 21 days. In conclusion, gelatin/alginate scaffolds, with or without HAP, fabricated by 3D plotting according to a predesigned CAD-model might be potential candidates for the repair of bony and chondral defects, especially in complex defect situations affecting the osteochondral tissue interface since biphasic scaffolds with a stable connection of the two parts can be easily fabricated by multi-channel 3D plotting.
1. Introduction
3D porous scaffolds provide a multi-functional platform in tissue engineering and regenerative medicine ensuring sufficient mechanical support, cell attachment and stimulation of new tissue growth in vivo. Material composition and structure are the two key factors which influence the properties of scaffolds, including the mechanical properties, degradation and cell responses.1,2 Therefore, in the past decades, most of the studies on engineered scaffolds were focused on components and structures. Lots of materials have been used to process into scaffolds as well as various methods and technologies have been developed to achieve suitable structures adapted to the respective tissue.Alginate, a biocompatible and biodegradable hydrogel whose sol–gel transition is induced by multivalent cations, is one of the widely used materials in tissue engineering.3,4 After crosslinking by Ca2+ ions, the scaffolds maintained their morphology and were characterized by a higher mechanical strength compared to scaffolds conventionally prepared from low concentration alginate sols. However, an obvious disadvantage of alginate scaffolds is their poor ability to support cell attachment and proliferation due to the lack of efficient sites for cell adhesion.5 Modification of alginate by introducing cell adhesion peptides (such as RGD peptides) is one option to circumvent this limitation.6 Another efficient method is to prepare composites by introducing other biomaterials which favor cell attachment to alginate-based scaffolds.
Gelatin is a natural material derived from collagen by hydrolysis and has a composition almost identical to that of collagen, the major extracellular matrix (ECM) component in animals.7 It contains some of the biological signals which promote cell adhesion, proliferation and differentiation such as the RGD sequence.8–10 Thus, the biological origin of collagen-derived gelatin makes this material an attractive choice for tissue engineering. Like alginate, gelatin has been applied for embedding of cells during scaffold fabrication, e.g. in a bioprinting process.11–13 In addition, gelatin was also reported to be used for preparation of scaffolds, by several methods, which were colonized with cells after their fabrication e.g. for cartilage and bone tissue engineering.14,15 Freeze-drying is the commonly used method to prepare gelatin or gelatin hybrid scaffolds,16–18 however, the pore parameters are hardly to control with this method, and the mechanical properties are generally insufficient for bone or cartilage tissue engineering. Electrospinning is another widely used method for generation of nanofiber gelatin scaffolds,19,20 but this method is limited with respect to the fabrication of porous 3D scaffolds with controllable macro-pores for cell penetration and new tissue ingrowth. In addition, the weak mechanical properties of such scaffolds are problematic for bone tissue engineering. Although gelatin is reported to be an excellent substrate for cell attachment, proliferation, and differentiation, the disadvantages of using gelatin as scaffold material in bone or cartilage tissue engineering are its low biomechanical stiffness and rapid biodegradation.21,22
The development of composite materials is a promising strategy to generate novel materials with improved qualities by combining different materials and their intrinsic properties. Consequently, scaffolds composed of gelatin with chitosan or alginate as second polymer and with calcium phosphate particles as inorganic component were fabricated. For example, Eslaminejad et al.23 prepared β-tricalcium phosphate–alginate–gelatin hybrid scaffolds with osteo-inductive properties via freeze-drying. Stancu et al.24 prepared gelatin/alginate hybrid sponges by freeze-drying and found that gelatin hydrogels with a controlled amount of alginate could be beneficial for induction of biomimetic mineralization. Xia et al.25 fabricated an injectable alginate/gelatin scaffold, which has been shown to promote bone healing in a rabbit calvarial critical-sized defect. Bernhardt et al.26 reported on the preparation and characterization of alginate–gelatin–hydroxyapatite composite scaffolds with orientated tube-like pores, possessing good cell attachment and osteogenic properties. However, nearly all of the alginate/gelatin or alginate/gelatin/calcium phosphate hybrid scaffolds reported so far were prepared on the basis of low concentration of the hydrogels (less than 4%) via conventional methods.
Rapid prototyping technologies such as 3D plotting play nowadays an important role for scaffold fabrication because of their great advantage regarding the realization of predesigned scaffold architectures.27,28 In the last years, various extrudable materials including polymer solutions, dispersions or melts, blends of different polymers or slurries of polymers and inorganic particles have been developed. However, the focus of using gelatin and/or alginate for rapid prototyping is mainly on biofabrication by developing cell embedding methods on the basis of low concentrated hydrogels. For instance, the application of an alginate dialdehyde–gelatin crosslinked hydrogel for bioplotting of cell-laden constructs was recently reported.29 However, due to their softness, hydrogels suitable for cell embedding have in most cases limitations regarding the realization of 3D scaffold designs. According to our best knowledge, there were no reports on fabrication of alginate/gelatin composite scaffolds through 3D plotting based on concentrated pastes.
Previously, we have developed a high concentration alginate paste which is suitable for CAD/CAM-based plotting into 3D scaffolds of predesigned architecture; even hollow fibers can be easily realized that opens up the possibility to generate tissue engineered constructs with a preformed vascular system.30–33 Based on that work, the aim of the present study was to develop novel high concentration plotting materials on the basis of gelatin and alginate for scaffold fabrication. Thus, the excellent plotting properties of alginate was combined with the quality of gelatin as cell substrate to allow the fabrication of predesigned 3D scaffolds of adequate mechanical strength, shape fidelity and cytocompatibility. Gelatin/alginate and gelatin/alginate/HAP composite scaffolds were fabricated via 3D plotting of the developed plotting materials; the resulting scaffolds were crosslinked in a dual process and characterized with respect to the compressive strength and modulus as well as the response of human bone marrow-derived mesenchymal stem cells (hBMSC) cultivated on the plotted scaffolds.
2. Experimental
2.1. Preparation of plotting pastes and scaffolds
For fabrication of the gelatin/alginate composite scaffolds, initially a gelatin solution was made by dissolving 16.7 wt% gelatin (bioreagent grade; Sigma Aldrich, Taufkirchen, Germany) in deionized water (preheated to 90 °C) under magnetic stirring (500 rpm and 90 °C). After the gelatin powder was dissolved completely, 7 g of the hot gelatin sol was mixed with 1 g alginate powder (Manugel®, pharma grade; ISP Alginates Ltd. Waterfield, Tadworth, U.K.); the mixture was stirred until a homogeneous paste has been formed. The paste, composed of gelatin/alginate in a mass ratio of around 54/46, was loaded into a cartridge and immediately used for plotting of scaffolds. For preparation of gelatin/alginate/HAP pastes, a gelatin sol with suspended HAP particles was generated in a first step by dissolving 11.8 wt% gelatin in deionized water (preheated to 90 °C) followed by adding 9.5 wt% HAP powder (Merck, Darmstadt, Germany) under magnetic stirring (500 rpm and 90 °C). The average size (d50) of the HAP particles was 2.3 μm and the size distribution (range of d10–d90) was ranging from 0.9 to 4.8 μm. After the gelatin was dissolved completely, 10.8 g of the homogeneous gelatin/HAP mixture was used in hot state to mix with 1 g alginate powder; finally the paste was composed of gelatin/alginate/HAP in a mass ratio of around 39/30/31. A second type of gelatin/alginate/HAP paste with a mass ratio of around 50/30/20 was prepared by mixing 1 g alginate powder with 11 g 15 wt% gelatin solution combined with 6 wt% HAP. After stirring to homogenous pastes, they were loaded into a cartridge and immediately used for scaffold plotting. As control, pure alginate scaffolds were fabricated by plotting an alginate paste, which was prepared by mixing 1 g alginate powder with 5 g deionized water.The scaffolds were fabricated by 3D plotting34 applying a three-channel plotting system developed by Fraunhofer IWS (Dresden, Germany) on the basis of the Nano-Plotter™ device from GeSiM (Grosserkmannsdorf, Germany).35 This system was equipped with a heating system that allows cartridge temperature control up to a maximal temperature of 120 °C. The working temperature was set in the range of 37 °C to 80 °C, adapted to the suitable dosing pressure and plotting speed. The pore geometry of the scaffolds was designed as square. After plotting, the scaffolds were transferred into a 1 M CaCl2 solution and incubated for 2 h at room temperature for crosslinking of alginate, followed by washing of the scaffolds with deionized water three times and drying at room temperature. Afterwards, the dry scaffolds were transferred into a solution of 2 wt% N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide (EDC; Fluka, Germany) and 0.25 wt% N-hydroxysuccinimide (NHS; Fluka) in 80 vol% ethanol and incubated for 12 h at room temperature for crosslinking of gelatin, followed by thorough rinsing in deionized water, 1 wt% glycine solution, and once again in deionized water. Scaffolds were dried at room temperature. Without specific description, all the scaffolds used in this work were crosslinked by CaCl2 and EDC in a two-step-process described above.
2.2. SEM and FTIR analysis
The prepared scaffolds were coated with carbon and characterized by SEM (scanning electron microscopy) using a Philips XL 30/ESEM, equipped with a field emission gun. Scaffolds of pure alginate, gelatin/alginate (54/46) and gelatin/alginate/HAP (39/30/31) after crosslinking and drying were analyzed by FTIR (fourier transform infrared) spectroscopy in transmission mode (Nicolet 380 FTIR Spectrometer, Thermo, USA). The samples were prepared by grinding particles of the scaffolds with KBr.2.3. Mechanical test
The compressive strength and modulus of plotted and crosslinked scaffolds (10 × 10 × 10 mm3) with a square macropore geometry were tested before (dry state) and after immersion in SBF (simulated body fluid) for 2 h at 37 °C (wet state) by using a mechanical testing machine (INSTRON 5566, Wolpert, Germany) equipped with a 10 kN load cell. The test was performed by compressing the scaffolds in z-direction at a constant rate of 1 mm min−1; 30% of compressive deformation was achieved for all scaffolds. Five samples were tested for each point.2.4. Cell culture
Scaffolds of pure alginate, gelatin/alginate (54/46) and gelatin/alginate/HAP (39/30/31) with a size of 6 × 6 × 3 mm3 were used for cell culture experiments. After sterilization by gamma-radiation at 25 kGy and pre-incubation in cell culture medium (Dulbecco's modified Eagle's medium low glucose supplemented with 9% fetal calf serum, 100 U ml−1 penicillin and 100 μg ml−1 streptomycin; all purchased from Biochrom, Germany) for 24 h, hBMSC (obtained from Cambrex, Walkersville, MD, USA) were seeded with an initial density of 5 × 104 per scaffold. The cell-seeded scaffolds were cultivated in an incubator at 37 °C and 5% CO2; cell culture medium was changed twice per week.After 1 and 21 days of culture, samples were prepared for cLSM (confocal laser scanning microscopy) analysis as described previously.35 In brief, samples were washed with PBS (phosphate buffered saline) trice, fixed with 4% formaldehyde in PBS, incubated with 3% BSA (bovine serum albumin; Sigma-Aldrich) in PBS for blocking the background and stained with Alexa Fluor 488® phalloidin (Invitrogen, Carlsbad, CA, USA) and DAPI (Sigma-Aldrich) to visualize actin cytoskeletons and nuclei of the cells. The cLSM characterization was performed with a Leica TCS SP5 (Germany). For SEM analysis, samples were taken after 1 and 21 days of culture, washed with PBS, fixed with 4% formaldehyde in PBS, washed with distilled water and dehydrated using a gradation series of ethanol/distilled water solutions. Critical point drying was performed with a CPD 030 apparatus (BAL-TEC AG, Liechtenstein). Dried samples were coated with gold and investigated using a Philips XL 30/ESEM with FEG (field emission gun) operated in SEM mode.
After 21 days of cultivation, viable cells on scaffolds were visualized by MTT staining as described previously.36 The samples were incubated in cell culture medium containing 0.5 mg ml−1 MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; Sigma-Aldrich) for 4 h and then observed by light microscopy (LEICA M205C with a DFC295 camera). In addition, an MTT assay was conducted after 1, 3 and 7 days of cultivation in order to evaluate proliferation of the cells on the plotted scaffolds. Briefly, 1 ml of the MTT solution (0.5 mg ml−1 in cell culture medium) was added to each sample. After incubation for 4 h, the solution was removed and 300 μl of DMSO (dimethyl sulfoxide) was added to solubilize the formazan product. An aliquot of 100 μl was transferred to a fresh 96-well plate; absorbance was measured at 590 nm in a microplate reader (Epoch™ microplate spectrophotometer, Bio Tek Instruments, USA). All the data are presented as optical density values minus the absorbance of blank wells.
2.5. Statistical analysis
All the data were expressed as mean ± standard deviation (SD) and were analyzed by one-way ANOVA. A p-value < 0.05 was considered statistically significant.3. Results and discussion
3.1. Preparation of the scaffolds
Gelatin is a temperature sensitive material. At sufficiently low temperature, the gelatin chains are able to form thermoreversible networks by associating helices in junction zones stabilized by hydrogen bonds.37,38 Consequently, a prepared pure gelatin sol formed stable gels at room temperature, which were unable to be extruded through plotting needles even by applying a very high dosing pressure and a big needle size (inner diameter: 1.4 mm). By increasing the temperature, the gelatin gel was reverse transformed into a solution which, however, was to liquid for plotting as it hampered the extrusion and deposition of stable rods to form uniform 3D porous structures. Liquid gelatin sol lacks sufficient mechanical strength to support the porous 3D structure of a plotted scaffold, it always led to deformation of the pores and collapse of the structure.39–41 Therefore, tissue engineering scaffolds consisting of gelatin or gelatin-based mixtures (such as gelatin/alginate mixtures) were mostly prepared by conventional methods, such as freeze-drying and particle leaching.42–44In this work, the advantage of gelatin to support cell adhesion was combined with the excellent properties of alginate regarding plottability: based on our previous work,31–33 high concentration mixtures of gelatin/alginate and gelatin/alginate/HAP were developed and tested for its plottability at different temperatures. Various ratios of gelatin and alginate were tested – those with excellent plotting properties were chosen for scaffold fabrication: 54/46 (gelatin/alginate) as well as 39/30/31 and 50/30/20 (gelatin/alginate/HAP).
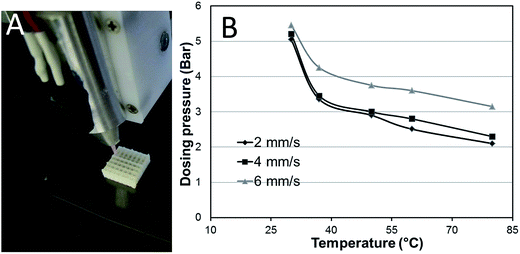
As shown in Fig. 1A, stable and homogeneous scaffolds can be easily plotted with the help of a heated cartridge based on the developed pastes. A study on the relationship between dosing pressure and working temperature by plotting a gelatin/alginate/HAP mixture (39/30/31) was performed and the data are presented in Fig. 1B. It can be seen that the required dosing pressure increased as the temperature decreased, however, when the temperature was lower than 37 °C, a very high pressure was required and even though, it became difficult to achieve homogeneous rods and stable scaffolds. Therefore, for plotting a homogeneous gelatin/alginate/HAP or gelatin/alginate scaffold, a temperature higher than 37 °C is required.
3.2. Characterization of the scaffolds
The structure of the plotted scaffolds was characterized by light microscopy and SEM. From the light microscopic images (Fig. 2A–C), it can be seen that the pores of the scaffolds were completely open and corresponded to the predesigned structure in all three scaffold types. The appearance of gelatin/alginate/HAP scaffolds was white, caused by the uniformly incorporated HAP particles, while that of pure alginate and gelatin/alginate scaffolds was brownish. SEM images (Fig. 2D–I) revealed that both the surface as well as cross-sections of alginate and gelatin/alginate were smooth and dense. In contrast, surface and cross-sections of gelatin/alginate/HAP scaffolds were characterized by a higher roughness which was observed in the whole scaffold indicating that the HAP particles were evenly distributed within the polymer matrix.The plotted scaffolds either consisting of gelatin/alginate or gelatin/alginate/HAP suffered from certain shrinkage due to the loss of water during drying. Nearly 37% and 47% shrinkage in X & Y and Z directions occurred for gelatin/alginate scaffolds (Fig. 3). However, despite of the large extent of shrinkage, the plotted scaffolds maintained their regular structure including the regular pore morphology. After immersing the dry scaffolds in aqueous solutions, the scaffolds reached the original size gradually.
 | ||
| Fig. 3 Shrinkage of plotted gelatin/alginate (54/46), gelatin/alginate/HAP (39/30/31, 50/30/21) and alginate scaffolds in X & Y and Z directions after drying at room temperature (n = 5; mean ± SD). | ||
FTIR spectra of alginate, gelatin and gelatin/alginate, gelatin/alginate/HAP are presented in Fig. 4. For alginate, absorption bands at 1639 cm−1 and 1423 cm−1 were attributed to the asymmetric and symmetric stretching vibration of COO– group, respectively. For gelatin, absorption bands at 1648 cm−1, 1557 cm−1 and 1247 cm−1 were attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–N stretching and N–H bending vibration, respectively. The wide adsorption band at around 3450 cm−1 was due to the stretching vibration of O–H. Compared to gelatin/alginate and gelatin/alginate/HAP, the adsorption bands of gelatin at 1648 cm−1 and 3450 cm−1 shifted to a lower wave number, suggesting intermolecular interactions between alginate and gelatin.45–47
O, C–N stretching and N–H bending vibration, respectively. The wide adsorption band at around 3450 cm−1 was due to the stretching vibration of O–H. Compared to gelatin/alginate and gelatin/alginate/HAP, the adsorption bands of gelatin at 1648 cm−1 and 3450 cm−1 shifted to a lower wave number, suggesting intermolecular interactions between alginate and gelatin.45–47
 | ||
| Fig. 4 FTIR spectra of gelatin (a), alginate (b), gelatin/alginate/HAP (39/30/31) (c) and gelatin/alginate (54/46) (d) scaffolds. | ||
3.3. Mechanical strength of the scaffolds
The compressive strength and modulus of the scaffolds were measured in dry and wet state at room temperature. The obtained data, presented in Fig. 5, demonstrates that plotted scaffolds had high compressive strength and Young's modulus in dry state. The compressive strength and modulus of gelatin/alginate/HAP (39/30/31) scaffolds were 77 ± 13 MPa and 268 ± 43 MPa, respectively, while, as control, the gelatin/alginate/HAP (39/30/31) scaffolds without EDC crosslinking had significant lower compressive strength (35 ± 13 MPa). Furthermore, the mechanical strength and modulus of gelatin/alginate scaffolds with or without HAP had no significant difference in dry state. A potential explanation for both, the high mechanical strength of the hydrogel-based scaffolds as well as the limited increase after addition of HAP particles, is the high polymer concentration that results in an extraordinary high stiffness of the crosslinked materials. In addition, after drying, the plotted scaffolds suffered from uniform shrinkage (around 40%) leading to a densification of the gelatin/alginate strands, while maintaining the scaffolds structure, which is connected with a further strong increase of the stiffness (SEM revealed the dense and compact bulk structures of the scaffolds). Furthermore, the Ca2+ crosslinked alginate and EDC crosslinked gelatin formed interconnected networks due to the intense physical intermolecular interactions between peptides and the polysaccharide.48 All these effects contributed to the very strong mechanical properties of the gelatin/alginate scaffolds in dry state.However, in wet state, the addition of HAP significantly increased the mechanical properties, although the compressive strength and modulus of all scaffold types decreased sharply in wet state compared to those in the dry state. Gelatin and alginate are both superior water adsorption materials, which are widely used as hydrogels. After incubation of the gelatin/alginate scaffolds in SBF or other aqueous solutions, the scaffolds were swollen due to the great water uptake, which then resulted in softening.48 Furthermore, the physical intermolecular interactions between alginate and gelatin were destroyed due to the strong water adsorption, and this is the main potential reason why the mechanical properties of alginate were higher than that of gelatin/alginate.
By comparing the data achieved in this work to those from other groups, it can be stated that the mechanical properties of the plotted gelatin/alginate scaffolds developed in this work were significantly better than those of gelatin/alginate scaffolds prepared by other groups with conventional methods. For example, Kim et al. prepared mixed gelatin/HAP scaffolds by freeze-drying and the scaffolds with 30% HAP and 84.6% porosity had an elastic modulus of 4.01 ± 0.39 (dry) and 1.12 ± 0.15 (wet) MPa.49 The modulus of our prepared scaffolds with a porosity of approximately 70% (calculated according to the uniform pore and pore wall size) was 67 times of that in dry state. The reason not only includes the lower porosity of our novel scaffolds, but more importantly is the regular structure and high concentration of the gelatin/alginate composites.
3.4. Cultivation of hBMSC on the scaffolds
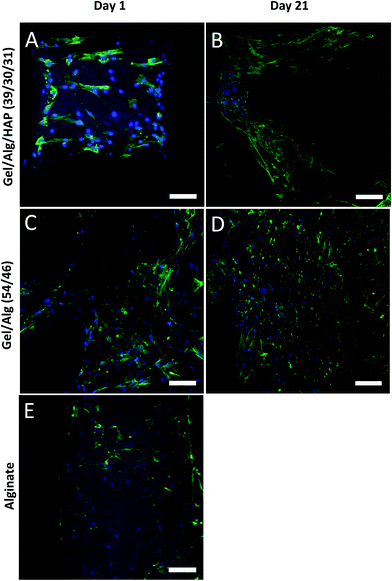
Cell adhesion, morphology and distribution on the scaffolds were observed by means of cLSM (Fig. 6) and SEM (Fig. 7); viability of the cells was studied by MTT staining (Fig. 8). The data indicated that hBMSC were able to attach to all three scaffold types: the cells showed spread morphology, even on pure alginate. However, a higher cell density was detected on the plotted gelatin/alginate scaffolds with or without incorporation of HAP compared to pure alginate scaffolds on day 1. In addition, cells on pure alginate were characterized by a patchy distribution. On day 21, cells covered the whole surface and even the pores of the gelatin/alginate scaffolds with or without HAP (Fig. 6 and 7). MTT staining of cell-seeded gelatin/alginate scaffolds after 21 days of cell culture revealed a uniform distribution of viable cells in the whole scaffold; even different layers were spanned by cells (Fig. 8).The incorporation of HAP in this system had limited positive effect: the gelatin/alginate composite without HAP seems to be much better for cell attachment and growth. Similar results were reported in other publications in which gelatin or gelatin/chitosan biopolymer scaffolds with and without HAP particles were examined.49–51 Potential reasons for the observed cell attachment and/or proliferation on the HAP containing composites are the higher surface roughness which is reported to possibly retard cell migration and proliferation,49 the change charge conditions due to the effective negative charge of the HAP particles which might influence cell attachment51 as well as a reduced accessibility of cell-promoting signals (such as RGD-sequences).
Gelatin, as mentioned, contains some of the biological signals which promote cell adhesion, proliferation as well as differentiation and is therefore an excellent candidate for preparation of tissue engineering scaffolds.52–54 In this study, we demonstrate that a high concentration gelatin/alginate composite is similarly suitable to support cell adhesion and proliferation (Fig. 9). On the other hand, the scaffolds prepared by 3D plotting have controlled and opened pores, which promote cell migration and proliferation.55,56 The microscopic images of the MTT stained scaffolds indicated a high cell viability in all scaffold regions as viable cells were observed covering all over the top surface as well as the inner parts of the gelatin/alginate scaffolds. In tissue engineering, one of the challenges is to ensure cell seeding and survival in the center of scaffolds. In most cases, without using of bioreactor systems, cells can only attach and survive on the surface of scaffolds especially for those prepared by conventional methods. In these cases, it is hard for cells to migrate and survive in the center of scaffolds due to the uncontrolled pore structure, i.e. with regard to interconnection and orientation, as well as limited oxygen and nutrients supply of the central regions. In this work, we showed that 3D plotted gelatin/alginate scaffolds had a completely controlled and open pore structure, which considerably contributed to the uniform cell colonization as well as survival and proliferation over 21 days.
4. Conclusion and outlook
The gelatin/alginate-based composite materials described herein are excellent candidates for tissue engineering approaches as they can be plotted into 3D scaffolds of predesigned architecture. The plotted scaffolds were stable after dual crosslinking with CaCl2 and EDC solutions; they possessed adequate mechanical strength and supported cell adhesion and proliferation. Based on these materials, 3D plotting can be used to fabricate advanced biphasic scaffolds which might be suitable for repair of osteochondral defects (Fig. 10). The bony part is composed of gelatin/alginate/HAP whereas the chondral part consists of gelatin/alginate. The integrity and stability between the two parts were achieved by dual crosslinking of alginate and gelatin. The scaffold fabrication method presented in this study provides a potential platform for repair of complex defects such as in osteochondral and periodontal tissues.Acknowledgements
This study was partly funded by the Saxon Ministry for Higher Education, Research and Arts (SMWK; contract no. 4-7531.60/29/24). The first author (YL) would like to thank the Shanghai Municipal NaturalScience Foundation (14ZR1445500), the Natural Science Foundation of China (Grants 31400807) for supporting this study.References
- V. Karageorgiou and D. Kaplan, Biomaterials, 2005, 26, 5474–5491 CrossRef CAS PubMed.
- S. Bose, M. Roy and A. Bandyopadhyay, Trends Biotechnol., 2012, 30, 546–554 CrossRef CAS PubMed.
- A. D. Augst, H. J. Kong and D. J. Mooney, Macromol. Biosci., 2006, 6, 623–633 CrossRef CAS PubMed.
- U. Schlossmacher, H. C. Schroder, X. Wang, Q. Feng, B. Diehl-Seifert, S. Neumann, A. Trautwein and W. E. G. Muller, RSC Adv., 2013, 3, 11185–11194 RSC.
- D. Suárez-González, K. Barnhart, E. Saito, R. Vanderby, S. J. Hollister and W. L. Murphy, J. Biomed. Mater. Res., Part A, 2010, 95, 222–234 CrossRef PubMed.
- J. Yu, K. T. Du, Q. Fang, Y. Gu, S. S. Mihardjs, R. E. Sievers, J. C. Wu and R. J. Lee, Biomaterials, 2010, 31, 7012–7020 CrossRef CAS PubMed.
- M. C. Gomez-Guillen, B. Gimenez and M. E. Lopez-Caballero, Food Hydrocolloids, 2011, 25, 1813–1827 CrossRef CAS PubMed.
- A. Ito, A. Mase, Y. Takizawa, M. Shinkai, H. Honda, K. I. Hata, M. Ueda and T. Kobayashi, J. Biosci. Bioeng., 2003, 95, 196–199 CrossRef CAS.
- C. H. Chang, H. C. Liu, C. C. Lin, C. H. Chou and F. H. Lin, Biomaterials, 2003, 24, 4853–4858 CrossRef CAS.
- W. Xia, W. Liu, L. Cui, Y. Liu, W. Zhong, D. Liu, J. Wu, K. Chua and Y. Cao, J. Biomed. Mater. Res., Part B, 2004, 71, 373–380 CrossRef PubMed.
- D. B. Kolesky, R. L. Truby, A. S. Gladman, T. A. Busbee, K. A. Homan and J. A. Lewis, Adv. Mater., 2014, 26, 3124–3130 CrossRef CAS PubMed.
- T. Billiet, E. Gevaert, T. De Schryver, M. Cornelissen and P. Dubruel, Biomaterials, 2014, 35, 49–62 CrossRef CAS PubMed.
- B. Duan, L. A. Hockaday, K. H. Kang and J. T. Butcher, J. Biomed. Mater. Res., Part A, 2013, 101, 1255–1264 CrossRef PubMed.
- S. M. Lien, L. Y. Ko and T. J. Huang, Acta Biomater., 2009, 5, 670–679 CrossRef CAS PubMed.
- X. Liu, L. A. Smith, J. Hu and P. X. Ma, Biomaterials, 2009, 30, 2252–2258 CrossRef CAS PubMed.
- M. B. Eslaminejad, H. Mirzadeh, Y. Mohamadi and A. Nickmahzar, J. Tissue Eng. Regener. Med., 2007, 1, 417–424 CrossRef CAS PubMed.
- H. J. Tseng, T. L. Tsou, H. J. Wang and S.-H. Hsu, J. Tissue Eng. Regener. Med., 2013, 7, 20–31 CrossRef CAS PubMed.
- X. Liu and P. X. Ma, Biomaterials, 2009, 30, 4094–4103 CrossRef CAS PubMed.
- Z. X. Meng, Y. S. Wang, C. Ma, W. Zheng, L. Li and Y. F. Zheng, Mater. Sci. Eng., C, 2010, 30, 1204–1210 CrossRef CAS PubMed.
- S. Panzavolta, M. Gioffre, M. L. Focarete, C. Gualandi, L. Foroni and A. Bigi, Acta Biomater., 2011, 7, 1702–1709 CrossRef CAS PubMed.
- P. Angele, R. Kujat, M. Nerlich, J. Yoo, V. Goldberg and B. Johnstone, Tissue Eng., 1999, 5, 545–553 CrossRef CAS.
- A. A. Al-Munajjed, M. Hien, R. Kujat, J. P. Gleeson and J. Hammer, J. Mater. Sci.: Mater. Med., 2008, 19, 2859–2864 CrossRef CAS PubMed.
- M. B. Eslaminejad, H. Mirzadeh, Y. Mohamadi and A. Nickmahzar, J. Tissue Eng. Regener. Med., 2007, 1, 417–424 CrossRef CAS PubMed.
- I. C. Stancu, D. M. Dragusin, E. Vasile, R. Trusca, I. Antoniac and D. S. Vasilescu, J. Mater. Sci.: Mater. Med., 2011, 22, 451–460 CrossRef CAS PubMed.
- Y. Xia, F. Mei, Y. Duan, Y. Gao, Z. Xiong, T. Zhang and H. Zhang, J. Biomed. Mater. Res., Part A, 2012, 100, 1044–1050 CrossRef PubMed.
- A. Bernhardt, F. Despang, A. Lode, A. Demmler, T. Hanke and M. Gelinsky, J. Tissue Eng. Regener. Med., 2009, 3, 54–62 CrossRef CAS PubMed.
- D. W. Hutmacher and S. Cool, J. Cell. Mol. Med., 2007, 11, 654–669 CrossRef CAS PubMed.
- B. Derby, Science, 2012, 338, 921–926 CrossRef CAS PubMed.
- T. Zehnder, B. Sarker, A. R. Boccaccini and R. Detsch, Biofabrication, 2015, 7, 025001 CrossRef PubMed.
- Y. Luo, A. Lode and M. Gelinsky, Adv. Healthcare Mater., 2013, 2, 777–783 CrossRef CAS PubMed.
- Y. Luo, A. Lode, F. Sonntag, B. Nies and M. Gelinsky, J. Mater. Chem. B, 2013, 1, 4088–4098 RSC.
- Y. Luo, C. Wu, A. Lode and M. Gelinsky, Biofabrication, 2013, 5, 015005 CrossRef PubMed.
- Y. Luo, A. Lode, C. Wu, J. Chang and M. Gelinsky, ACS Appl. Mater. Interfaces, 2015, 7, 6541–6549 CAS.
- R. Landers and R. Mülhaupt, Macromol. Mater. Eng., 2000, 282, 17–21 CrossRef CAS.
- A. Lode, K. Meißner, Y. Luo, F. Sonntag, S. Glorius, B. Nies, C. Vater, F. Despang, T. Hanke and M. Gelinsky, J. Tissue Eng. Regener. Med., 2014, 8, 682–693 CrossRef CAS PubMed.
- K. Schütz, F. Despang, A. Lode and M. Gelinsky, J. Tissue Eng. Regener. Med., 2014 DOI:10.1002/term.1879.
- C. Michon, G. Cuvelier, P. Relkin and B. Launay, Int. J. Biol. Macromol., 1997, 20, 259–264 CrossRef CAS.
- M. Curcio, U. G. Spizzirri, F. Iemma, F. Puoci, G. Cirillo, O. I. Parisi and N. Picci, Eur. J. Pharm. Biopharm., 2010, 76, 48–55 CrossRef CAS PubMed.
- R. Landers, U. Hübner, R. Schmelzeisen and R. Mülhaupt, Biomaterials, 2002, 23, 4437–4447 CrossRef CAS.
- X. Wang, Y. Yan, Y. Pan, Z. Xiong, H. Liu, J. Cheng, F. Liu, F. Lin, R. Wu, R. Zhang and Q. Lu, Tissue Eng., 2006, 12, 83–90 CrossRef CAS PubMed.
- P. S. Maher, R. P. Keatch, K. Donnelly, R. E. Mackay and J. Z. Paxton, Rapid Prototyping Journal, 2009, 15, 204–210 CrossRef.
- A. T. Neffe, B. F. Pierce, G. Tronci, N. Ma, E. Pittermann, T. Gebauer, O. Frank, M. Schossig, X. Xu, B. M. Willie, M. Forner, A. Ellinghaus, J. Lienau, G. N. Duda and A. Lendlein, Adv. Mater., 2015, 27, 1738–1744 CrossRef CAS PubMed.
- F. Han, Y. Dong, Z. Su, R. Yin, A. Song and S. Li, Int. J. Pharm., 2014, 476, 124–133 CrossRef CAS PubMed.
- J. Lacroix, E. Jallot and J. Lao, Chem. Eng. J., 2014, 256, 9–13 CrossRef CAS PubMed.
- Z. Dong, Q. Wang and Y. Du, J. Membr. Sci., 2006, 280, 37–44 CrossRef CAS PubMed.
- S. Teng, J. Shi, B. Peng and L. Chen, Compos. Sci. Technol., 2006, 66, 1532–1538 CrossRef CAS PubMed.
- Y. Li, H. Jia, Q. Cheng, F. Pan and Z. Jiang, J. Membr. Sci., 2011, 375, 304–312 CrossRef CAS PubMed.
- I. C. Stancu, D. M. Dragusin, E. Vasile, R. Trusca, I. Antoniac and D. S. Vasilescu, J. Mater. Sci.: Mater. Med., 2011, 22, 451–460 CrossRef CAS PubMed.
- H. W. Kim, J. C. Knowles and H. E. Kim, J. Biomed. Mater. Res., Part A, 2005, 72, 136–145 CrossRef PubMed.
- M. Peter, N. Ganesh, N. Selvamurugan, S. V. Nair, T. Furuike, H. Tamura and R. Jayakumar, Carbohydr. Polym., 2010, 80, 687–694 CrossRef CAS PubMed.
- C. Isikli, V. Hasirci and N. Hasirci, J. Tissue Eng. Regener. Med., 2012, 6, 135–143 CrossRef CAS PubMed.
- S. R. Gomes, G. Rodrigues, G. G. Martins, M. A. Roberto, M. Mafra, C. M. R. Henriques and J. C. Silva, Mater. Sci. Eng., C, 2015, 46, 348–358 CrossRef CAS PubMed.
- H. M. Powell and S. T. Boyce, J. Biomed. Mater. Res., Part A, 2008, 84, 1078–1086 CrossRef CAS PubMed.
- B. Lei, K. H. Shin, Y. H. Koh and H. E. Kim, J. Biomed. Mater. Res., Part B, 2014, 102, 1528–1536 CrossRef PubMed.
- J. M. Sobral, S. G. Caridade, R. A. Sousa, J. F. Mano and R. L. Reis, Acta. Biomater., 2011, 7, 1009–1018 CrossRef CAS PubMed.
- F. Pati, T. H. Song, G. Rijal, J. Jang, S. W. Kim and D. W. Cho, Biomaterials, 2015, 37, 230–241 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |