BiVO4/MIL-101 composite having the synergistically enhanced visible light photocatalytic activity
Abstract
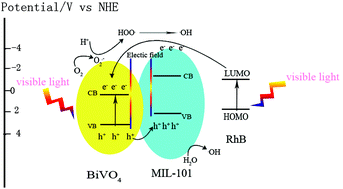
In this study, a novel composite containing a Cr based metal–organic framework (MOF), MIL-101 and BiVO4 was successfully synthesized by hydrothermal method, and was fully characterized by X-ray diffraction, transmission electron microscopy, thermogravimetric analysis, UV-vis diffuse reflectance absorption spectra and photoluminescence emission spectra. Moreover, the photocatalytic activities of BiVO4/MIL-101 composite and the pure materials were evaluated by measuring the degradation of Rhodamine B under visible light. The results show that the composite exhibits better photocatalytic activities than pure materials, which can be ascribed to the big adsorption capacity of MIL-101 and the enhanced separation of photogenerated charge carriers from assembly of MIL-101 on BiVO4. The synergistic effect between BiVO4 and MIL-101 was evaluated by the proposed synergistic factor, which is bigger than 1 for various ratios of BiVO4 to MIL-101, suggesting there exists positive interactions between pure materials for enhancing photocatalytic activities.

 Please wait while we load your content...
Please wait while we load your content...