Preclinical appraisal of terbutaline analogues in precipitation of autism spectrum disorder
Abstract
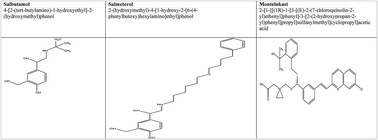
Terbutaline is a β2 agonist used in the clinical management of asthma and as a tocolytic agent during pregnancy. In the recent past, the preterm use of terbutaline has been shown to hasten autistic-like symptoms in offsprings. For this reason, the present study was carried out to understand the effects of pharmacological analogues of terbutaline (salbutamol, salmeterol and montelukast) in the progression of ASDs in experimental animals. Pregnant rats were treated with salbutamol (10 mg kg−1, sc), salmeterol (10 μg kg−1, sc) and montelukast (10 mg kg−1, sc) and the offsprings were scrutinized for behavioral, biochemical, neuro-inflammatory and histopathological changes. The offsprings from the rats treated with salbutamol and montelukast were determined to be closely associated with various symptoms of ASDs.

 Please wait while we load your content...
Please wait while we load your content...