An overview of radioisotope separation technologies for development of 188W/188Re radionuclide generators providing 188Re to meet future research and clinical demands
Ashutosh Dash
a and
F. F. (Russ) Knapp Jr
*b
aIsotope Production and Applications Division, Bhabha Atomic Research Centre (BARC), Mumbai 400 085, India
bEmeritus, Medical Isotopes Program, Isotope Development Group, Oak Ridge National Laboratory (ORNL), Room 203, MS 6229, Bldg. 4501, PO Box 2008, 1 Bethel Valley Road, Oak Ridge, TN 37831-6229, USA. E-mail: knappffjr@ornl.gov; Fax: +1-865-574-1900
First published on 14th April 2015
Abstract
The role of the tungsten-188/rhenium-188 (188W/188Re) generator system to provide the no-carrier added (NCA) 188Re therapeutic radionuclide for applications in nuclear medicine and oncology is well established. The evolution and successful use of the 188W/188Re generator in nuclear medicine has resulted from new discoveries and innovations from separation science along with technological advances which have broadened the scope and utility of 188W/188Re generators. Nonetheless, there are still additional opportunities for improvements and innovations in separation science which will undoubtedly continue to provide improvements in 188W/188Re generator technology. In this review, we discuss the reported separation technologies such as the adsorption-type systems which have been traditionally used as well as emerging separation technologies which have the potential for further development of 188W/188Re generator systems. This article also outlines the comparative advantages and disadvantages of various key separation technologies. Further, the regulatory challenges, the impact on 188W/188Re technology with the emergence of professionally run central radiopharmacies, and the role of automation are discussed.
1. Introduction
Reference to radionuclide generators is well established in the literature and refers to radioisotope separation systems which provide radioisotopes for a broad array of both diagnostic and therapeutic applications in nuclear medicine, oncology, cardiology and related fields.1–9 In most of these systems, the shorter half-life daughter radioisotope is repeatedly removed after generation by radioactive decay from the longer lived radioactive parent. The technologies involved in the development and application of these systems include radiochemistry and separation science. Radionuclide generators represent important capabilities for nuclear medicine practice in particular, and have contributed significantly for advances in this field. The well-established role of the 188W/188Re generator provides no-carrier added (NCA) 188Re (half-life 16.9 hours) on-demand from the decay of 188W (half-life 69 days) in a cost-effective manner for a variety of clinical applications in radionuclide therapy (RNT). Since the generator is essentially an in-house radioisotope production system used on demand, an important advantage is the lack of reliance on local accelerator or reactor production capabilities.10–17 Interest in the use of 188Re for RNT is mainly attributable to its favorable nuclear decay characteristics, which include a reasonable half-life, emission of high energy beta radiation (Eβmax = 2.118 MeV), emission of a 155 keV γ-ray (15%) which is suitable for gamma camera imaging, and versatile chemical properties which are suitable for the preparation of a wide variety of radiopharmaceuticals.17–19 The harnessing of nuclear and chemical properties of 188Re in convergence with advances in molecular and cellular biology, the development of complementary technologies for targeting agents and the increased interest in the use of unsealed therapeutic radioactive sources, have focused on the use of 188Re for various clinical applications. In addition, convenient generator availability is expected to broaden the palette of 188Re-labeled radiopharmaceuticals for RNT in the foreseeable future.17–23 There is a steadily expanding list of 188Re labeled radiopharmaceuticals that are currently in clinical study stages, or which can potentially be evaluated for therapy.17–26 Widespread applications of 188Re have already stimulated progress and helped the RNT field move forward.While development and use of the 188W/188Re generator encompasses technologies from many disciplines, its development, availability and use are based on technical issues associated with separation science. The remarkable progress for use of 188Re available from the 188W/188Re generator has in part resulted from advancements in separation science. Advances in 188W/188Re generator technology continue to be based on innovations in separation technologies. Since the field of separation science is subjected to continuous evolution, any revolutionary breakthrough on this subject not only represents an important driving force but also will have a widespread and far reaching impact. The utility of a separation system is dictated by its ability to provide 188Re with acceptable very low levels of 188W in a seamless manner.
The tremendous prospects associated with use of the 188W/188Re generator, along with the challenge of providing 188Re of requisite quality for a variety of therapeutic procedures, continue to lead to fascinating research and development of innovative separation strategies. With view to obtain 188Re in an acceptable chemical form amenable for the formulation of current generation of 188Re-labeled pharmaceuticals, a large number of separation strategies have been exploited. New separation technologies and new concepts are in the early stages of development.
In order to sustain the important use of 188Re for RNT, it is of utmost importance to nurture emerging separation technologies in an appropriate manner to facilitate their transition from laboratory research to the clinical setting. The recent surge of interest in the use of 188Re in RNT has been the motivation to provide this detailed review on recent advances in separation science and how they can be exploited for further improvements in the development of 188W/188Re generator technologies. As emerging separation techniques move from the laboratory to generator applications, the possible impacts of these new technologies will become evident over time. As the scope of separation science is expanded, these technologies will acquire value added capabilities for restructuring the 188W/188Re generator technology to address present needs and to meet future research and clinical demands.
The aim of this article is thus to provide an overview of current separation technologies which are currently in use, or which have made substantial progress and are likely to be materialized in the foreseeable future. In the following sections we discuss an overview on the different types of 188Re separation techniques, their principles, relative strengths and weakness, contemporary status and apertures to the near future. Given the multidisciplinary nature of this field, speculative options reported mainly of academic interest are not included and the authors apologize for possible oversights of important contributions. This review is intended to serve as a resource to offer an impetus for further development, not only for separation chemists, but also for scientists and technologists involved in 188W/188Re generator development. Expectations, capabilities, constraints, and gratifications involved in developing the 188W/188Re generator prototypes for various clinical applications are discussed.
2. Importance of 188Re-labeled radiopharmaceuticals and the 188W/188Re generator for radionuclide therapy
Before discussing 188W/188Re the separation strategies in detail, it is important to discuss 188Re-based radiopharmaceuticals and the importance of the continued availability of 188W/188Re generators. In recent years, there have been unprecedented interests in the use of 188Re for RNT by virtue of its favorable nuclear characteristics and versatile chemistry. There are many practical and economic advantages for using 188Re in RNT and the remarkable progress in the development and use of 188Re-labeled agents over the last decade has resulted from many attributes. A key characteristic of this generator ensures cost-effective on-demand availability of NCA 188Re from the 188W/188Re generator in a clinical setting. The highly energetic β− radiations emitted by 188Re (Eβ(max) = 2.118 MeV) have the advantage of relatively long-range tissue penetration (∼13 mmmax in soft tissue) and effective radiation dose distribution in large size tumors. Rhenium-188 decay is accompanied by a predominant 155 keV γ-emission (15%), which can be detected by γ-cameras, for imaging, biodistribution, and absorbed radiation dose estimates. The 16.7 h half-life of 188Re is optimum for preparing radiopharmaceuticals either in a hospital radiopharmacy or centralized radiopharmacy setting, performing quality control, administration, time for target tissue accumulation, and image collection, yet short enough to minimize toxicity risks during therapy and to often allow out-patient therapy. The therapeutic effects of the high energy β− emission are relatively rapid, due to the short half-life (16.7 hours) which results in a high dose-rate, compared with many other therapeutic beta emitting radionuclides of current interest. Owing to the short half-life and low abundance of gamma irradiation, hospitalization of patients undergoing therapy is usually not necessary, since outpatient use of many 188Re-labeled agents is thus possible. According to the U.S. Nuclear Regulatory Committee (NRC) guidelines (Regulatory Guide 8.39—Release of patients administered radioactive materials), up to 29 GBq (790 mCi) of 188Re can be administered to outpatients, which is much higher activity than doses allowed for many other therapeutic agents. The versatile chemistry of rhenium emerges from the existence 8 possible Re oxidation states, which provide the scope for attachment to a variety of targeting molecules with specific characteristics. Such a possibility provides great versatility for development of a range of 188Re-labeled therapeutic agents. Also importantly, use of this 188Re also offers the scope for preparation of ‘matched pair’ theranostic applications in association with use of the 99mTc diagnostic radioisotope. The increasing commercial availability of various freeze dried “kits” (i.e. substrate preparations in vials ready for radiolabeling) offers the versatility of preparing a wide variety of 188Re therapeutic agents.While the therapeutic applications of 188Re are quite promising and some are already well established (i.e. bone pain palliation, non-respectable liver cancer therapy, arterial restenosis therapy after angioplasty, etc.), dependence on the availability of 188W/188Re generators is an important aspect. The generators must provide 188Re with the required quality to satisfy the current radiolabeling chemistry requirements which represent an important capability for radiopharmacy use of 188Re. As the potential of 188Re labeled compounds for RNT is increasingly recognized, a range of 188W/188Re generators for widespread use in daily nuclear medicine routine have been developed and commercially introduced. The use of 188W/188Re generators in nuclear medicine is thus very attractive, since onsite availability of NCA 188Re on a day-to-day basis is ensured, obviating the need for ready access to radionuclide production facilities. The 69.4 day half-life of the 188W parent with the in-growth of 188Re after elution allows use of the generator for an extended time period, of at least 6 months or even longer, depending on the 188Re activity levels required for a specific application. The 16.7 h half-life of 188Re permits generator elution, for examples, at 24 hour intervals, to obtain about 50% yields of 188Re. This generator system also represents an attractive option for countries having no research reactor production facility and/or situations where other therapeutic radioisotopes, such as 177Lu and 90Y, fro example, are unavailable or too expensive. The 188W/188Re generator can also provide 188Re during periods when a reactor is not in operation (e.g., due to maintenance or repairs) owing to its ability provide 188Re at any time, integrating and optimizing the daily clinical throughput.
Therapeutic applications of 188Re in nuclear medicine have depended on the availability of 188W/188Re generators, and availability of this system in conjunction with the well-established coordination chemistry of 188Re has been the bases for the development of 188Re radiopharmaceuticals. Both 99mTc and 188Re are members of the group 7 transition metal series, and have almost identical ionic radii owing to lanthanide contraction. Although Re(VII) complexes are more prone to oxidation, both Re and Tc form analogous complexes, many of which have similar stability, with similar chemical structures which differ only in the metal center, and exhibit analogous “in vivo” biological behavior. The [188ReO4]− anion is obtained from the 188W/188Re generator and is usually reduced to lower oxidation states for the preparation of radiopharmaceuticals. The final oxidation state depends on the reducing agent, nature of the chelator and reaction conditions. Despite chemical similarities, the standard reduction potentials of technetium and rhenium are markedly different. On an average, the E0 values of redox reactions involving technetium process are about 200 mV higher than that of the corresponding rhenium process. As a result, reduction of [188ReO4]− appears to be much more difficult than that of [99mTcO4]− and therefore requires more drastic reduction conditions than for [99mTcO4]−.27 This more difficult reduction of perrhenate has emerged as the major factor which poises challenges for development of new 188Re-radiopharmaceuticals. In order to circumvent such a limitation, “expansion of the coordination sphere” concept was developed and implemented for the preparation of the [188ReO(DMSA)2] (DMSA = dianionic dimercaptosuccinic acid) complex.28 Such a strategy can presumably be extended to other systems.
The fundamental requirement for preparation of 188Re-based radiopharmaceutical is availability of requisite generally structurally modified biomolecules that bind the reduced 188Re perrhenate anion ion in a stable coordination complex so that these molecules can be properly directed to a desirable molecular target in vivo. In light of the requirements to introduce 188Re into a variety of targeted biomolecules, the direct, indirect and integral radiolabeling approaches have been evaluated. Direct labeling techniques are limited to compounds which themselves are ligands or which contain structures such as disulphide bonds, for instance, which can be reduced to provide the –SH chelation sites for 188Re. Indirect labeling is used for preparation of most 188Re-labeled biomolecules and involves the initial attachment of an exogenous chelator. Integral labeling is used for the radiolabeling of small molecules in which the metallic radionuclide serves to link two parts of a biomolecule together in forming the radiolabeled complex.29 Although a detailed discussion concerning 188Re radiopharmaceuticals is beyond the scope of this article, the development and use of these agents have been widely reported elsewhere.16–26 The in vivo application of key 188Re radiopharmaceuticals for a variety of therapeutic procedures include 188Re-dimercaptosuccinic acid (188Re-DMSA) for the treatment of medullary carcinoma; 188Re-hydroxyethylidine diphosphonate (186Re-HEDP) for the treatment of bone pain due to skeletal metastases; mixtures of lipiodol and 188Re-labeled liphophilic agents (188Re-labeled HDD/lipiodol and DEDC/lipiodol) for the treatment of inoperable hepatocellular carcinoma (HCC); 188Re-labeled particulate/colloids for radiosynovectomy of medium size joints; 188Re labeled peptides in receptor-mediated radiotherapy; 188Re labeled antibodies in radioimmunotherapy (RIT); 188Re agents for intravascular radionuclide therapy (IVRNT) and 188Re patches for treatment of non-melanoma skin cancer.
While the cost of a 37 GBq (1 Ci) 188W/188Re generator is about 20 times higher than that of a typical 37 GBq (1 Ci) 99Mo/99mTc generator, the unit dose costs of 188Re are generally similar to 99mTc, owing to a much longer useful shelf life of the 188W/188Re generator of >6 months, which is approximately 20 times longer than that of the 99Mo/99mTc generator. Interest for use of the 188W/188Re generator for therapeutic applications rests on the opportunity to elute 1.85–2.59 TBq (50–70 Ci) of 188Re from a 37 GBq (1 Ci) 188W/188Re generator over a period of six months. In light of the perceived need for optimal use of 188Re obtained from a 188W/188Re generator to minimize 188Re unit dose costs, institutions must optimize generator use by performing multiple applications such as targeted therapy of cancer, bone pain palliation and radiation synovectomy, etc. The success of 188W/188Re generator for therapy has been based on the optimal use of 188Re to derive its overall potential in RNT.
3. Production of 188W
The evolution and continued success for broad use of the 188W/188Re generator has largely resulted from availability of the required activity levels and high quality 188W of adequate specific activity. In order to further demonstrate the proven benefit of 188Re in RNT, it is important to have assured access to appropriate production as well as target processing technologies that will provide 188W in the desired quantity and quality to fabricate clinical scale 188W/188Re generators. While fission production route is a successful paradigm to obtain high specific activity/no-carrier-added 99Mo, such a possibility for the production of 188W is precluded, since 188W is not produced through fission of 235U. For this reason, neutron irradiation of the 186W target is a necessity. The neutron activation routes that lead to the production of 188W along with its decay scheme are depicted in Scheme 1. The 186W undergoes two consecutive neutron captures to provide 188W.Table 1 summarizes the nuclear constants available from literature for radionuclides involved in the 188W production chain. Although “direct” neutron activation of 186W permits production of 188W, this route results in the production of 188W with relatively low specific activity 188W as a consequence of the double neutron production process,17 modest thermal reaction cross-section values, target self-shielding and product burn-up during neutron irradiation.31–33 With a view to produce 188W of adequate specific activity and required purity amenable for generator fabrication, pertinent factors are discussed below in detail.
| Nuclide | Decay constant, λ (s−1) | Cross-section, σ (b) | Values for resonance integral, I (b) |
|---|---|---|---|
| 186W | — | 37.9 | 485 |
| 187W | 8.09 × 10−6 | 64.0 | 2760; 10 |
| 188W | 1.16 × 10−7 | 12 | 0; 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000; 1.4 000; 1.4 |
| 187Re | — | 76.4 | 300 |
| 188Re | 1.13 × 10−5 | <2 | — |
In order to optimize the specific activity of 188W, the prospect of irradiating the target at the highest available thermal neutron flux is necessary. Since the production yield for a successive double neutron capture process is a function of the square of the neutron flux (ϕ), increasing the flux by only one order of magnitude will double the 188W activity produced. The calculated specific activity of 188W obtained at various neutron flux values is shown in Fig. 1, illustrating the dramatic increase in specific activity with increasing thermal neutron flux. From a practical perspective, a minimal thermal neutron flux of ∼1 × 1015 neutrons per cm2 per s is required to produce a specific activity of about 1 Ci 188W g−1 of 186W, which is generally adequate for preparation of the alumina based, chromatographic type 188W/188Re generator system. It is evident from the data presented in this figure that reactors having thermal neutron flux values of less than 5 × 1014 n per cm2 per s are generally not amenable for the production of 188W, since the lower specific activity results much higher generator elution volumes which subsequently decrease the specific volume (mCi mL−1) of 188Re, as discussed in more detail later.
 | ||
| Fig. 1 Calculated specific activity of 188W at various thermal neutron flux f = ϕth/ϕEp = thermal to epithermal neutron flux ratio. | ||
With an aim to produce 188W of sufficient specific activity for generator use as well as to minimize the radiation field contributed by other radioactive isotopes of W in the production chain (185W, 187W), the use of 186W enriched targets is essentially a necessity because of the relatively low natural abundance (28.6%) of 186W. It is crucial to re-cover the expensive non-activated enriched 186W target material from spent generators for recycling to decrease the target cost over many production campaigns.34
The neutron burn-up cross-section for the 188W(n,γ)189W nuclear reaction is another factor that must be considered because of its influence on reduction of 188W production yields. In order to minimize contribution of this factor, the time period for neutron irradiation must be judiciously optimized.35 The issue of self-shielding of target also must be taken into account while selecting the chemical form of the target, as this factor also decreases the specific activity.33 While the use of enriched high temperature sintered metallic 186W target constitutes a successful paradigm in terms of enhancing target density,34 loading and 188W production capability per target precludes an increase in specific activity because of target self-shielding. The specific activity obtained in this case is considerably less (20–25%) than the specific activity of the irradiated granular/powder enriched 186W target.30 The current status of reactor production and processing of 188W has been summarized in detail in a document recently published by the International Atomic Energy Agency (IAEA).36 As described in this report, 188W with the highest specific activity most suitable for the fabrication and use of the alumina-based 188W/188Re generators is only available from the two high flux research reactors available at the Reactor Institute for Atomic Research (RIAR), Dimitrovgrad, Russian Federation (SM Reactor) and at the Oak Ridge National Laboratory, Oak Ridge, TN, USA (High Flux Isotope Reactor, HFIR).
3.1 Post-irradiation target processing
Although 186W targets for reactor irradiation can consist of a variety of chemical forms, the granular/powder/pellets of 186W-enriched metal and tungsten oxide targets have been most widely used since simplified processing can be achieved by simple dissolution in sodium hydroxide solution with heating (powder/granular), or following high temperature oxidation to the oxide. While the use of powder enriched 186WO3 targets was the practice for 188W production in the HFIR at the Oak Ridge National Laboratory (ORNL) in Oak Ridge, TN, USA, for many years for fabrication of the 188W/188Re alumina-based generators,30–32 the pressed and sintered enriched 186WO3 discs have more recently become the target of choice at ORNL.33 The targets are sealed in Suprasil quartz ampoules prior to encapsulation in the T6061 high purity aluminum capsules used for reactor insertion and irradiation. If metallic granular/powder or pressed/sintered enriched 186W metallic targets are used, it is essential to initially heat the irradiated target material to 750–800 °C in a quartz furnace in a stream of air for conversion to tungsten oxide for subsequent dissolution in base, as shown in Fig. 2.The processing of neutron irradiated granular 186W metal targets is conducted in a quartz glass vessel which essentially involves heating to 750–800 °C in a quartz furnace while passing a stream of air over the target material. In this process, 188W metal is converted into 188WO3. The contaminating levels of most of the 191Os on heating with oxygen converted into volatile, 191OsO4 (melting point 30 °C, boiling point 130 °C). During the oxidation of W metal, the exhaust goes through a series of trap (two traps containing 0.1 N NaOH + charcoal filter unit) connected in-line of the heating system to retain sublimed 191OsO4 and 188WO3 if formed due to raise in furnace temperature >800 °C. Such a strategy is attractive since the metallic target is not only converted to soluble tungsten oxide, but this process also efficiently removes the majority of the 191Os radionuclidic impurity as 191OsO4, which is swept away from the target by the stream of air for subsequent trapping in base. During the conversion, it is essential to maintain the temperature of the furnace in the range of 750–<800 °C as oxidation of tungsten metal is accompanied by subsequent volatilization of the 188WO3 above 800 °C. On heating with oxygen, the metallic 192Ir contaminant is converted to 192IrO2, which volatilizes above 1130 °C. Therefore, the low levels of the 192IrO2 impurities remain with the 188WO3 product. The resulting 188WO3 in the quartz glass vessel is subsequently dissolved in 6 M NaOH where it is converted into sodium tungstate solution. The sodium tungstate stock solution is not purified further, since the possible presence of low amounts 192Ir impurities present in the 188Re generator eluents used for radiopharmaceuticals preparation has been shown to be inconsequential. Such a strategy is attractive since the metallic target is not only converted to soluble tungsten oxide, but this process also efficiently removes the majority of the 191Os radionuclidic impurity as OsO4, which is swept away from the target by the stream of air for subsequent trapping in base.
At RIAR, tungsten oxide targets isotopically enriched with 186W up to 96% are encapsulated in the quartz capsule, sealed in the titanium ampoule and irradiated in the SM Reactor at the RIAR in Dimitrovgrad, Russian Federation for 18–24 days.36–38 In order to allow short-lived 187W to decay, targets are cooled for 5–7 days and processed in a hot-cell. The quartz capsule is crushed and tungsten oxide is dissolved in 2 M NaOH by heating. In order to ensure complete dissolution of the irradiated tungsten oxide, freshly prepared sodium hypochlorite is added to the reaction mixture. During heating, radioactive gases containing 191Os in the form of OsO4 are allowed to pass through a series of traps containing hydrochloric acid solution of thiourea (Trap 1) and mixed sodium alkali and ethanol (Traps 2 and 3). To remove fragments of quartz capsule, the resulting solution is filtered through filter paper and evaporated to minimum volume. To insure the solution is free from 187Re, it is passed through an anion-exchange column packed with Dowex-1 resin in NO3− form. As the target is dissolved in sodium hydroxide solution, quartz is also partially dissolved and contaminated tungsten solution with silicates. In order to make the solution free from silica as well as anionic impurities such as chloride, chlorate and perchlorate ions, it is passed through column containing Dowex-1 anion-exchange resin in Cl− form. The solution of (presumably) pertungstic acid is then evaporated to incipient dryness, reconstituted with sodium hydroxide (close to stoichiometric ratio) and subjected to quality evaluation. A schematic diagram of the apparatus used at RIAR for post irradiation 186W target processing is shown in Fig. 3 and the corresponding flow sheet is shown in Scheme 2.
3.2 Tungsten-188 backup production capabilities
While 188W production is reported to be primarily carried out at the ORNL HFIR and the SM Reactor at the RIAR, as for all reactors, these facilities are vulnerable to disruptions in production schedules due to the predictable and unpredictable issues associated with reactor maintenance, refueling, upgrades, regulatory compliance and requirement for other programs. It is thus imperative to have backup production capabilities for 188W, which requires long irradiation periods in lower thermal neutron flux reactors. Table 2 summarizes examples of those reactors with sufficiently high thermal neutron flux that have been used for 188W production of and has the potential to produce 188W as backup having sufficient specific activity amenable for the preparation of column chromatographic 188W/188Re generators.| Reactor | Institution | Reported/projected specific activity (Ci 188W g−1 W) | Remarks | |
|---|---|---|---|---|
| 1 | HFIR | ORNL, Oak Ridge, TN, USA | 4–5 Ci, one cycle. 8–9 Ci, two consecutive cycles | Routine production since 1986 |
| 2 | SM3 | Research Institute for Atomic Reactors (RIAR), Dimitrovgrad, the Russian Federation | ∼5 Ci for several ‘mini-cycles’ | Routine production since about 1986. Backup production for ORNL |
| 3 | BR2 | SCK·CEN, Mol, Belgium | ∼1 Ci, 20+ d cycle; cycle length varies | Experience since about 2001. Backup production for ORNL |
| 4 | ATR | Idaho National Laboratory (INL), Idaho Falls, USA | ∼0.5 Ci, one cycle expected | Production not yet initiated |
It is pertinent to point out that collaboration between ORNL and SCK·CEN in 1998 had successfully demonstrated that the BR2 Reactor in Mol, Belgium could be utilized for 188W production. Production of 188W in the BR2 Reactor was carried out in the central beryllium plug H1 where the thermal neutron flux peaks to a value of 1 × 1015 n per cm2 per s.39 The high-density pressed metallic 186W targets (97% enriched) fabricated at ORNL were provided to Mol for irradiation and the irradiated targets then shipped back to ORNL for subsequent processing and fabrication of generator which were then shipped to IAEA-supported research sites in developing countries. The specific activity of BR2-produced 188W was about ∼37 GBq (1 Ci) g−1 using a 20+ day irradiation cycle.
3.3 Separation processes for the 188W/188Re generator
Selection of an appropriate separation technology based on a number of considerations is among the critical factors contributing to the remarkable success for use of 188Re available from the 188W/188Re generators. A primary consideration is the ability to effectively separate 188Re in reproducible yield from 188W with the highest possible decontamination factor. In this regard, the radionuclidic, radiochemical and chemical purities of 188Re should be within acceptable limits, and when required, within the pharmacopeia acceptable range. Also, the radioactive concentration (RAC) of 188Re should be adequate to perform radiolabeling with a wide range of biomolecules. Another key issue is that the process should be facile, robust, devoid of any violent chemical reactions, labor-non-intensive, and have high throughput capabilities. The efficiency of the separation process in terms of purity as well as 188Re yield should remain unaltered for long term operation and the separation process employed should permit the scope of 188W “recharging” of the generator periodically with minimal chemical manipulation. Another key factor requires that the chemical reagents used for separation should have adequate radiation stability, since radiation instability will not only diminish separation efficiency but may also contaminate 188Re with degradation products. The separation process should also generate minimum quantities of radioactive waste and human intervention should be minimal to diminish radiation dose to operating personnel and the generator system should be amenable for safe operation. Another practical key element would insure recovery of no-activated enriched 186W target material34 from generators which have exceeded their shelf-life for recycling and target preparation.While governed by a number of factors, the selection of a separation process is primarily aimed at simplification of the overall separation procedure to provide 188Re of required quality and optimum yield in a seamless manner throughout the shelf life of the 188W/188Re generator. Overviews of the principles regarding radioactive equilibria, in-growth and equilibrium of the daughter radionuclide with parent radionuclide, have been elaborately discussed in detail in recent reviews.40–42 In-growth of the 188Re in 188W/188Re generator is continuous, and once the 188Re activity of the daughter is recovered from the 188W/188Re equilibrium mixture, 188Re activity increases until its activity level reaches a maximum and is in equilibrium with 188W activity as depicted in the Fig. 4.
The in-growth and separation of 188Re can be continued as long as there are useful activity levels of 188W available. Separation may be performed any time before equilibrium is reached, and the activity levels of 188Re recovered will depend on the time elapsed since the last separation. The generator reaches a value of about 62% of the equilibrium after 24 hours; therefore, the daily elution will provide approximately 50% of the 188Re that would be available at equilibrium, and can be used for the preparation of 188Re based therapeutic agents on a daily basis.
In the following sections, an overview on the different types of separation techniques, conceptual components, their utility, relative strengths and weakness are summarized, and the potential for their application in the development of 188W/188Re generator is discussed. Among the main factors contributing to the success of a separation process lies in its ability to provide 188Re in a facile manner and maximized yield. Amenability for safe operation either on the small scale as individual units at hospital-based radiopharmacies or on the large scale in central radiopharmacies is also an important criterion for selecting a particular separation processes. While some of the separation technologies discussed in this manuscript were not initially intended for use in conjunction with the 188W/188Re generator, they were later evaluated for further development of potential 188W/188Re generator prototypes.
3.4 Column chromatography
The solid–liquid separation technique involves partition of 188W and 188Re achieved by means of differences in their affinities toward the adsorbent. This represents the classic generator design used in many systems (i.e. 99Mo/99mTc, 82Sr/82Rb, 90Sr/90Y, etc.), where the parent radionuclide is bound tightly to a solid adsorbant, and elution with an appropriate liquid phase removes the desired daughter radionuclide. Overall, selectivity of the adsorbent determines the efficiency of 188W adsorption from solution, the ease with which 188Re can be subsequently removed from the adsorbent, and the degree (α = separation factor) to which 188W and 188Re can be separated from one another. In order to adsorb 188W strongly on the adsorbent to insure reproducible 188Re elution using a suitable eluent, the prospect of selecting an adsorbent having a high distribution coefficient (KD) for 188W with a low KD for 188Re is required. While performing separation, a number of aspects that must be considered, include column type, column bed dimension, pre-treatment of column bed, 188W loading technique, sample throughput, and reagents used for 188Re elution. The most widely used adsorbant for the 188W/188Re separation is acidic alumina.Among the various separation technologies introduced for development of 188W/188Re generator, for many practical reasons the column chromatography technology has significantly dominated the field. A major advantage of the column chromatographic technique is its operational simplicity which not only reduces the radiation exposure to the operating personal but also precludes potential contamination during set-up and operation. The system should be operated repeatedly throughout the shelf-life of the generator and in contrast to other separation techniques, product reproducibility in terms of 188Re purity and elution yield on continual use are well defined for the column chromatographic technique. In addition, acceptable radionuclidic (RN), radiochemical (RC) and chemical purity of 188Re are attainable. Use of this technique also generates negligible quantity of radioactive waste and offers the possibility of recovering the enriched 186W target from the spent column.
Although use of alumina column chromatographic technique for the development of the 188W/188Re has been broadly evaluated in the clinical setting, it does have some weaknesses, which include the limited W mass binding capacity of the alumina adsorbent, which dictates the use of high specific activity 188W for optimal output. Also, the kinetics of 188W column adsorption using this technique is generally slow, but the elution of 188Re is rapid. The narrow range pH control of the eluent is required to achieve acceptable purity of 188Re. The utility of the column chromatographic technique for long term operation of 188W/188Re generator is dictated by the chemical and radiation stability of the adsorbent.
The utility of column chromatography technique for separation of 188Re from 188W for radionuclide generator development is well established and a variety of column matrixes have been evaluated. The first work on chromatography 188W/188Re generator was reported in 1956 by Huffman et al.43 in which Dowex-1 in chloride form was used as the column matrix and the elution of 188Re was performed using 1.5 M HCl. While the reported procedure was primarily aimed to meet the need of radiochemists for the ready separation of rhenium from tungsten, this gave birth to the development of 188W/188Re generator. In another system developed in 1969, tungsten fluoride was absorbed on an anion exchanger and 188Re was eluted with perchloric acid.44 Although these studies had been productive, susceptibility of the organic resin to radiation damage emerged as the major impediment that had limited their applicability for 188W/188Re generator development. Radiolytic damage inflicted by organic resins can lead to decrease in 188Re yields, increased 188W breakthrough and decreased flow through the chromatographic column. In order to circumvent such drawbacks, the scope of using alternative column matrices based on inorganic backbones that are stable under radiation was felt necessary and subsequent developments were subsequently based on inorganic exchangers.
In Oak Ridge, Hayes and Rafter had initially developed an 188W/188Re generator prototype using zirconium oxide45,46 and later this theme was extended by Lewis and Eldrige.47 While the use of zirconium oxide represents an early a successful attempt to preclude radiation damage, both the systems used methyl ethyl ketone (MEK) to elute 188Re which was then evaporated and reconstituted with NaCl solution for radiolabeling. It is interesting to note that these early investigators had developed these systems to obtain the longer-lived 188Re radioisotope as an alternative to 99mTc for diagnostic imaging, rather than therapeutic use, and the issues associated with radiation dose had not been considered. In 1975, Malyshev et al.48 also reported a 188Re generator based on zirconium oxide in which 188Re was eluted from the column with distilled water with radionuclidic purity more than 99.99% with 50% yield. Ehrhardt et al. had also reported an improved version of 188W/188Re generator based on zirconium oxide that was proposed for biomedical applications.49 This procedure was later modified by Knapp and colleagues at ORNL,50 directed towards the development of a1.332 GBq (36 mCi) generator using commercially available zirconium oxide Bio-Rad HZO-1 (100–200 mesh).
In 1970, Klofutar et al. reported a radiochemical separation for rhenium using an alumina column.51 Subsequently, in 1972, Mikheev et al., in the former U.S.S.R., had developed a system in which tungsten was adsorbed on an alumina column as phosphotungstate and rhenium was eluted by saline at pH 3.52 The first medical 188W/188Re generator based on alumina column was reported by N. Botros et al.53 in which 188Re was availed in NaCl solution. These investigators studied sorption behavior of tungsten and rhenium on anion exchange resin, charcoal and alumina and found that the system based on alumina was the most promising systems to be used for the preparation of 188W/188Re generator. They had suggested a recommended procedure in which 188Re could be obtained in NaCl. Investigators in the former U.S.S.R.54 had also carried out the systematic evaluation of 188W sorption on alumina from several acidic aqueous phases and sodium sulfate, sodium chloride and sodium nitrate. Alumina was identified as the best adsorbent with best stability in a pH range of 1–6. While 0.15 M saline was the eluant of choice, sodium sulfate in concentrations less than 0.035 M also eluted perrhenate in good yields. Maximal yields for elution of perrhenate with saline were 70–90% of 188 Re. The generators exhibited reproducible performance over a 6–12 month period, although no values of 188W parent breakthrough were reported.
The alumina based 188W/188Re generator that became widely used in a variety of clinical trials developed by Knapp et al. at ORNL was further modified to provide the 188Re as sodium perrhenate with negligible 188W breakthrough with high radioactive concentration (RAC)10,11,55,56 amenable for the preparation of current generation of 188Re radiopharmaceuticals. Over the years, the use of ORNL 188W/188Re generator in various institutions has demonstrated consistently high 188Re yields accompanied by low 188W parent breakthrough during periods of several months. A typical clinical-scale ORNL 188W/188Re generator [loaded with 188W > 37 GBq (1 Ci)] initially provides more than 750 mCi (>75% yield) of 188ReO4− at equilibrium (30–35 mCi mL−1) or approximately 500 mCi (20–25 mCi mL−1) for initial sequential daily elutions. The yields of 188Re as well as low 188W breakthrough remain unaltered during at least 60 days of operation.17
3.5 Post elution concentration of 188Re
The 188Re eluent volume of depends on the mass (grams) of the generator column adsorbent which in turn is inversely proportional to the specific activity of 188W. Owing to the relatively limited capacity of bulk alumina as well as specific activity of 188W, large amounts of the alumina adsorbent (>9 g) are required for the preparation of traditional clinical-scale 37 GBq (1 Ci) 188W/188Re generators. This limitation thus requires a large volume of solution (>15–20 mL) for 188Re bolus elution. A 37 GBq alumina based 188W/188Re generator provides 188Re of with a RAC of 1.85–3.7 GBq mL−1; which is generally not suitable for the direct formulation of radiopharmaceuticals for clinical use. While the alumina based 188W/188Re generator constitutes a successful paradigm for availing 188Re for routine clinical applications, the limited capacity of the alumina emerged as the major impediment that continues to limit efforts to achieve the necessary RAC of 188Re for radiolabeling. With a view to reduce the size of the columns and at the same time to avail acceptable radioactive RAC of 188Re, use of high specific activity 188W represents a necessity.However, in light of the perceived need to concentrate 188Re solutions obtained from 188W/188Re generators to the high specific-volume solutions, post-elution concentration (PEC) of the eluate was developed as a useful relatively simple strategy to obtain much higher concentrations of 188Re. Use of simple evaporation of the eluate solution is precluded because the high salt concentration in the final solution is unacceptable for most biologically active compounds. In the quest for an innovative concept within the frame work of column chromatographic technique, elegant PEC procedures involving the use of tandem type generators consisting of an alumina based generator column with one or two columns had been developed by Knapp and his team at ORNL.57–61 This method consists of passage of the generator eluent through an ion exchange column to trap ‘no-carrier-added (NCA)’ levels of the 188Re perrhenate anion, followed by elution in a small volume of normal saline. The scope of using PEC is attractive not only to ensure long term exploitation of the generator but also paved the way for using the 188W/188Re generator prepared from medium specific activity 188W. Three different principle methods of 188Re post elution concentration have been described and implemented in generator prototype systems.
3.6 Use of IC-Ag and Sep-Pak Accell Plus QMA anion exchanger column
This elegant technique developed at ORNL uses a Maxi-Clean IC-Ag; Ag+ form cation exchanger cartridge (Alltech Associates, USA) in tandem with a Sep-Pak Accell Plus QMA anion exchange cartridge (Waters Corporation, Milford, USA) as the first method of post elution concentration.57–59 The 188Re eluate in normal saline solution obtained from a 188W/188Re generator is passed through an Alltech IC-Ag+ cation exchange cartridge to remove the macroscopic Cl− ions by AgCl precipitation. This results in the 188Re perrhenate eluate free of chloride anion which is then passed through the small Sep-Pak Accell Plus QMA anion exchange cartridge to retain the perrhenate anion. Subsequent re-elution with a very small volume (1 mL) of normal saline provides the highly concentrated 188Re solution ready for radiolabeling. The schematic representation of this strategy is depicted in Fig. 5. This method provides 188Re yield in 79 ± 3% with a concentration factor of about 10. The system is amenable for automation as all three columns (the generator itself, the chloride removal column and the concentrating column) are in the ‘on-line’ mode and all flows are switched with three way valves.57–59 This tandem column method has been used for most of the reported clinical applications with 188Re-labeled agents. | ||
| Fig. 5 Schematic diagram of post elution concentration set up using IC-Ag and Sep-Pak Accell Plus QMA anion exchanger column. | ||
3.7 Use of IC-H plus cation (Dowex-H)-anion (QMA light) column system
Another PEC procedure developed by ORNL is based on the removal of a salt component with a cation exchange resin followed by selective sorption of perrhenate ions with the anion exchange resin.60,61 In this procedure reported by Guhlke et al. from ORNL, the primary 188Re ammonium acetate eluate is allowed to pass through the column containing a strongly acidic cation exchanger where the cation (e.g. ammonia) is replaced by protons of the resin, and thus the solution leaving the column contains a weak acid (e.g. acetic and perrhenic acid, or pertechnic acid in the case of the 99Mo/99mTc generator system). The effluent from the cation exchanger column is then passed through a column containing a strongly basic anion exchange resin which retains the perrhenate (or pertechnetate ions in the case of the 99Mo/99mTc generator system). In this column, the weak acetic acid cannot compete with perrhenate ions owing to its low degree of dissociation. Rhenium-188 retained by the anion exchange column can be eluted using NaCl solution or DM water with high concentration factors. The final eluate contains small quantities (<0.1%) of the acid anion (e.g. acetate) used for production of the primary eluate. The presence of the acetate ion in eluate is not an impediment; however, as sodium acetate is often used as a component of solutions used in synthesis of 188Re labeled radiopharmaceuticals. While the method is effective in providing 188Re of acceptable concentration, a disadvantage of this procedure is the lower elution yield values of 188Re when ammonium acetate rather than 0.15 M saline is used as an eluent. Schematic representation of PEC procedure based on this concept is illustrated in Fig. 6. | ||
| Fig. 6 Schematic representation of PEC procedure based on the removal of a salt component with a cation exchange resin followed by selective sorption of perrhenate ions with the anion exchange resin. | ||
3.8 Use of a single column of DEAE cellulose
Another PEC procedure developed by investigators at the Board of Radiation and Isotope Technology (BRIT) in India, is based on the elution of the 188Re perrhenate in acetate buffer and sorption of perrhenate ions on a small anion exchange column containing diethyl amino ethyl (DEAE) cellulose anion exchanger column to trap 188ReO4−. Subsequently, 188Re not retained by the DEAE column is eluted using small volume of normal saline.62 In this method, the radiochemical purity of 188ReO4− is >98% with a 188W breakthrough less than 10−3%.Other PEC technologies for concentrating generator derived 188Re eluate exploiting solvent extraction63 and electrochemical techniques64 have also been reported over the last years. Given the simplicity of adsorbing low RAC 188Re parent and the ease of availing acceptable RAC by simple elution, the PEC technique has attracted considerable attention among users. While the PEC procedure using column chromatography technique has been prolific and has drawn widespread acceptance, conspicuous harnessing of the PEC technique with automation will not only revitalize but could foster the sustainability of this novel concept. This strategy has already made some progress65,66 which is an encouraging step forward which must be further exploited to expand the scope of this approach.
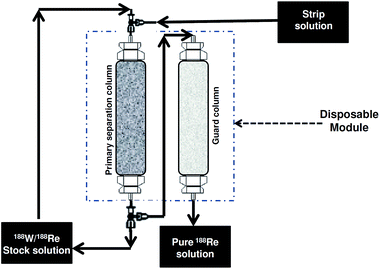
3.9 Multicolumn selectivity inversion (MSIG)
Another notable incremental innovative development within the realm of column chromatographic technique has been the multicolumn selectivity inversion (MSIG) generator.67,68 The method essentially comprises of two step adsorption and elution cycles involving two chromatography columns known as primary separation column (PSC) and guard column (GC). These two columns reside at the heart of MSIG generator, each of which function in a starkly different manner to deliver clinical grade 188Re. The PSC column contains an adsorbent having high affinity for 188Re and a low affinity for 188W whereas the adsorbent in GC has high affinity for 188W and a low affinity for 188Re. In this method the 188W/188Re solution is allowed to pass through PSC where in 188Re is adsorbed leaving 188W in the effluent. It is important to note that this strategy is similar to 188Re trapping on a micro column, but an important advantage is the use of a liquid flow system which is readily automated and the retention of 188W in solution, to minimize adsorbant radiation damage. In an attempt to make the PSC free from trace concentration of 188W and to ensure near complete recovery of 188W, the PSC is washed with a small volume of rinse solution. Both the effluent and washing of 188W are collected and stored for regrowth of 188Re for subsequent recovery. Rhenium-188 adsorbed in the PSC is eluted and passed through the guard column (GC) to free it from 188W. It is pertinent to point out that no chemical adjustment is made to the solution before passing through the GC. The efficiency of MSIG is governed by the selection of adsorbent for PC and GC. As the adsorbent used in PSC and GC have different selectivity for 188W and 188Re, and these selectivity are inverted, the acronym MSIG has been assigned.Since the shelf life of 188W/188Re generator can be greater than 6 months, the adverse effects of radiolytic degradation of column matrix as a result of β− radiation can lead to a deterioration of the separation efficiency and breakthrough of 188W. In this context, the shrewd choice of using MSIG minimizes radiolytic damage to the column matrix because the 188W “stock” solution is stored in solution and the 188Re is only adsorbed to the resin in PSC for a short time of only several minutes. The two most important factors for the design of a 188W/188Re generator system based on MSIG are the high 188Re elution efficiency and minimal 188W breakthrough. The advantages offered by the use of MSIG include the prospect of using low specific activity 188W, since the process is equally effective for both high specific activity and low specific activity 188W. The pairing PSC and GC afford not only high decontamination but also ensures overall chemical and radionuclidic purity of 188Re since there is only very negligible 188W breakthrough. The high separation efficiency of the system permits the possibility of using small column size owing to the NCA characteristic of 188Re which in turn facilitates recovery in a small volume eluate solution. Furthermore, the generator system may have a longer shelf than the conventional chromatographic column generator designs, since this system precludes radiation degradation of column matrix due to the relatively minimal contact time of 188W with the adsorbent.
The in-growth of 188Re in an aqueous acidic solution rather than in the adsorbent is a positive feature which offers the scope of recovering theoretical quantities of 188Re. Use of this system offers the scope of continuous recharging of the generator at periodic interval and the activity level can be scaled up or down as on demand. Use of this system also offers the prospect of quantitative recovery of the non-activated 186W enriched target material. An important capability, not unique to this system, is the amenability for automated modules commensurate with the hospital and central radio-pharmacy requirements. As with the introduction of all such systems there have also been some concerns expressed for use of this technique which include the longer processing times required to obtain 188Re compare to conventional column chromatographic methods. Also, the utility and success for routine use of the MSIG system depends on a number of factors that include the selection of the adsorbent material for PSC and GC which requires tedious experimental analysis and tests. While the concept of utilizing PSC and GC is appealing, use of such a system in a hospital radiopharmacy set up is a major deterrent unless the system is automated.
A 188W/188Re generator system based on PSC containing aqueous biphasic extraction chromatographic (ABEC®) resin and GC containing alumina has been developed.69 The solution containing 188W in 6 M NaOH, with the 188Re at some level of ingrowth is passed through the PSC which selectively retains the 188Re while passing the 188W into the recovery vessel. From NaOH solutions, perrhenate is strongly retained by the ABEC® resin, while 188W exhibit very little retention. With a view to make the column free from NaOH and to minimize the losses of 188W, PSC is flushed with air. The 188Re is eluted from the PSC using normal saline and is passed through GC containing alumina. The selective retention of the NCA 188Re by ABEC® resin offers the prospect of using a very small PSC and the same time tender recovery of the 188Re in relatively small volume of saline solution, irrespective of the specific activity of the 188W. The use of the GC seemed to be attractive for favorable outcome owing to its ability to retain any 188W breakthrough that may be present in 188Re. A general schematic diagram of a 188W/188Re generator system based on the MSIG concept is depicted in Fig. 7.
The MSIG concept has merit and holds great promise owing to its potential to use low specific activity 188W produced from medium flux nuclear reactor for the preparation of 188W/188Re generator system and maturation of this groundbreaking technology is expected to reinforce the 188W/188Re generator technology of today and tomorrow. The MSIG radionuclide generator concept has undergone a transformation over the past decade owing to technology maturation and an increased interest for commercialization for development of a functional 188W/188Re generator system. Use of this system may be poised to shape the future 188W/188Re generator system by combining MSIG technologies with the power of automation. The progressive fusion of MSIG technology with automation would not only ensure a sustained growth and future expansion of 188W/188Re generator system but also anticipated to empower future developments. This strategy has already made some progress70–75 but must be hastened further to expand its scope. A fully integrated computer controlled operation system with suitable features is an achievable objective as the basic automation technology already developed for ‘RadioGenix™’ for 99Mo/99mTc generator by North Star Medical Radioisotopes, LLC can be easily extended into the 188W/188Re generator owing to their chemical similarities.76 The system would allow automated approach for 188Re separation and preclude manual intervention from operators and is considered as an important step toward achieving the goal of establishing a reliable and commercially viable source of 188Re using low specific activity 188W. This will help improve patient care, advance important medical research on 188Re pharmaceuticals and alleviate regulatory concerns.
3.10 Gel-based 188W/188Re generator systems
An innovative paradigm without radically breaking away from the column chromatography concept is the ‘gel generator’. The genesis of the ‘gel-type generator’ was developed as a possibility to homogeneously incorporate 188W into an insoluble inorganic matrix and subsequent packing in a column chromatography, instead of adsorption on an adsorbent within a chromatography column. While the gel generator concept was originally developed for the 99Mo/99mTc generator,77 it was extended for use with 188W/188Re generators. While the choice of an insoluble inorganic matrix depends on a number of factors, it primarily exploits the differences in the solubility of 188WO3− and 188ReO4−. The chemical characteristic of the matrix should be such to offer nearly quantitative elution of 188Re with minimal release of 188W breakthrough. With a view to use 188W gel as a supporting matrix in a chromatography column from which 188Re formed from radionuclide decay of 188W can be obtained, the gel must be dried and broken up into free-flowing small granules. The real advantage of this approach also lies in its ability to use low specific activity 188W produced in a medium flux reactor. The gel generator concept possesses several advantages which include offering the scope of using low specific 188W produced in medium flux reactors. These systems provide 188Re of acceptable purity in 0.9% NaCl solution amenable for the preparation of 188Re labeled radiopharmaceuticals.Despite these very positive features, however, the concept of a gel-type 188W/188Re generator system has limitations which include the requirement of a capital intensive shielded facility to undertake cumbersome chemical processing of radioactive materials which not only result in relatively high manufacturing costs but also poses significant potential radiological safety risks. Also, the multistep complex gel processing procedure is intricate due to several factors influencing the gel characteristics which in turn affect final performance. The non-reproducibility of transforming the gel to small particles for generator loading is a factor which greatly affects the generator elution characteristics. This system is not only manufacturer unfriendly, but also could lead to inter batch variation. In order to undertake regular production of 188W gel, it is essential to have an adequate number of well-trained qualified skilled personnel. There is also a need to obtain adequate purity as well as RAC of 188Re from the generator which requires a post elution processing step.
In order to take advantage of the potential of gel generator concept for the development of 188W/188Re generator, substantial efforts had been focused towards the use of zirconium(IV) and titanium(IV) salts in which 188W co-precipitated with zirconium(IV) ion to form a polytungstate of metal ion as gel. The 188W/188Re generator based on Zr tungstate were made by dissolving neutron-irradiated 186W tungsten trioxide in a strong base and using the zirconyl salt in acid solution under optimum conditions to quantitatively precipitate 188W, followed by filtration, washing with water, alcohol, diethyl ether, optimal drying of gel cake and conversion to granules serving as a suitable column matrix.78–81 An alternative to the zirconium tungstate gel is the titanium tungstate gel. Dadachova and coworkers reported the preparation of a titanium-tungstate gel using natural tungsten followed by neutron irradiation of the preformed gel in a moderate flux reactor.82,83 It was reported that the “post-formed” approach permits fabrication of gel generators demonstrating excellent 188Re elution performance. The reported results suggest that titanium-tungstate gel generators with 188Re elution performance and elution yields very close to those of conventional alumina 188W/188Re generator could be obtained. Although the reported method obviously held promise as an alternative approach, the presence of curie-levels of 185W formed as a result of neutron activation of natural tungsten poses a major challenge during processing of the target material and during generator preparation and subsequent elution of the generator. According to an investigation carried out by Dadachova on gel generators based on Zn, Co, Ni, Mn and Pb tungstates as potential supports, Zn tungstate was found to be best in term of 188Re yield and purity.83
It is of interest to note that the preparation of the 188W/188Re gel generator involves certain considerations quite different from those in conventional column chromatography generator. With a view to obtain 188W gels consistently having the desirable characteristics of high 188Re release and minimal 188W breakthrough, a number of experimental parameters such as reactant concentration, alkalinity/acidity of reactant solution, solution concentrations, the order of reactive agent addition, mixing time and temperature, pH of gel formation, filtration rate, controlled drying and pulverizing of the gel cake, etc., required optimization. While the passage of an aqueous eluent (typically either purified water or normal saline) through a column of tungstate gel releases 188Re, an additional mini-column of alumina is required to remove impurities (e.g. 188W, Zr, Ti) in the eluate. Since the gel can withstand thermal (wet steam) autoclaving, the generator can be directly sterilized. The need for technically intense operations in hostile radiation environments and the lack of convenient methods for the recovery of valuable enriched 186W from the spent gel generator for recycling (subsequent neutron irradiation), have evidently been the major roadblocks in the path of the success of the gel technology. Despite the impressive progress in the development of 188W/188Re gel generators, the potential of this innovative concept has failed to live up to its initial promise and optimism and commercialization of this technology has evidently not moved forward.
3.11 Column chromatography using high capacity adsorbents
Another strategy within the realm of column chromatography that has captured the attention of investigators is the development of high capacity adsorbents capable of adsorbing much larger quantities of 188W than alumina which is conventionally used for the preparation of 188W/188Re generators. Consequently, a great deal of effort has been expended in recent years towards the development of high capacity adsorbents. The prospect of using high capacity adsorbents is appealing since these systems offer the scope of using low specific activity 188W without significantly altering the design the current generation of 188W/188Re generators.In an attempt to take advantage of the potential of high capacity adsorbents for the preparation of 188W/188Re generators, Matsuoka et al. have developed a poly zirconium compound (PZC).84 On a similar theme, Le Van So et al. developed polymeric titanium oxychloride sorbent (PTC).85 Adsorption capacities of up to 520 mg of tungsten per gram of PZC and 515 mg of tungsten per gram of PTC, were reported. Elution yields greater than 80% were achieved with both the PZC and the PTC sorbents. Tungsten-188 breakthrough values of 0.015% and elemental tungsten breakthrough of less than 5 mg mL−1 were found in the 188Re eluate. The performance of the PTC sorbent closely resembled that of the PZC sorbent, except that the breakthrough of 188W was higher. The PTC column also required a smaller volume of saline to elute 188Re. Since low specific activity 188W was used in these studies, the 188Re obtained required post-elution concentration. While preliminary results are promising, the inherent drawbacks of these systems (PZC, PTC) include the need for loading of radioactive 188W solution into the sorbent by batch process, heating the solution for an extended period of time to realize optimum capacity owing to slow kinetics of sorption and requirement of sophisticated remote handling processing facilities to avoid radiation exposure. Even if the generator could be manufactured, the operating performance thereof deteriorates with time. While preliminary results are promising, the generator systems are yet to be evaluated at high activity level operations and other harsh conditions typically encountered in fabrication of clinical scale generators. The promise of manufacturing 188W/188Re generator using these sorbents in a clinical context has not yet been realized, and the current statuses of these generators are unknown.
The prospect of using hydroxyapatite synthesized using the sol–gel method as an alternative yet effective high capacity adsorbent for the development of 188W/188Re generators has also been explored owing to its ability to adsorb about 0.9% weight of tungsten.86,87 The highest average elution efficiency of 188Re with 0.9% NaCl solutions at pH 6 was found to be 80–70%. While the system was effective to provide 188Re in normal saline, the release of phosphate ions due to dissolution of hydroxyapatite emerged as the major impediment which, however, can be circumvented by washing the generators with 0.01 M CaCl2 or 0.004 M NaH2PO4 after each elution with 0.9% NaCl solutions. Considerable R&D is required to assess the potential of this approach adaptable to the preparation of clinical 188W/188Re generators.
The scope of using gel metal-oxide composites has also been examined, but with limited success.88,89 Organic–inorganic hybrid mesoporous anion-exchange resins based on either pure silica or organ silica support materials were prepared and tested for the adsorption of perrhenate (ReO4−) anions in aqueous solutions but with limited success.90,91 While the utility of using organic–inorganic hybrid adsorbents is in its nascent stage and there are considerable challenges to develop 188W/188Re generators, this class of adsorbent has the potential to offer new opportunities.
A synthetic alumina functionalized with a sulfate moiety has also been developed as the column material of 188W/188Re generators.92 This material is synthesized by a sol–gel processing. The maximum capacity of the adsorbent for tungsten is reported to be higher than 450 mg g−1. The efficacy of the sorbent was demonstrated by developing a 1 Ci 188W/188Re generator. Elution efficiency of 188Re was 70–90% by using 5 mL of the saline solution. The ratio of 188W/188Re in the eluted solution is 0.002–0.003%, and the 188Re obtained from this generator required post-elution purification to reduce the 188W level. The current status of these studies is unknown and the scope of using this adsorbent is relatively more appealing in this regard, especially if adequate attention is focused on evaluating the usefulness of this adsorbent under high radioactive doses and other harsh conditions typically encountered in the operation of 188W/188Re generators.
Within all the unknowns and uncertainties related to capacity of the adsorbents and their ability to separate 188Re of acceptable quality, as well their relative trade-offs, promise to develop a 188W/188Re generator using this class of adsorbent amenable for use in a clinical setting has not yet been fulfilled, and the future prospects of this class of adsorbent do not appear promising.
3.12 Column chromatography using nanomaterial adsorbents
While the use of high capacity adsorbents for the development of a 188W/188Re generator constitutes a positive step in the right direction, there are limitations on the density of surface active sites, activation energy of adsorptive bonds and the mass transfer rate of the bulk materials. In this premise, the scope of using nanomaterial based adsorbent is not only an interesting prospect but also expected to offer new opportunities because they exhibit the unique physiochemical properties due to the quantum size effect that cannot be anticipated from their bulk counterparts. Summarily, nanomaterials have many special attributes which include the high percent of atoms on the surface, which have fewer neighbors than atoms in the bulk. Because of this lower coordination and unsatisfied bonds, surface atoms are less stabilized than bulk atoms. The unsaturated surface atoms can come in contact and therefore bind with other atoms/ions. As a result, these atoms possess unique adsorption properties due to distributions of reactive surface sites and disordered surface regions. The ability to manipulate the surface chemistry allows a new level of selectivity control that is expected to offer the scope for attaining sufficient specificity to adsorb 188W.The favorable adsorption kinetics permits high flow operation. The smaller dimensions of nanoparticles can produce short diffusion paths to the material interior and thus offer more favorable mass transfer reactions. In addition to these factors, the high adsorption capacity and reversibility of adsorption process which permit recovery of enriched target material are important. These systems are mechanically strong and robust to withstand attrition, erosion and crushing during long term column operation and possess adequate chemical and radiation stability to ensure long life or durable utilization. Moreover, the availability of synthetic procedures to undertake large scale manufacturing in a cost effective manner are available.
These attributes of nanomaterials have generated widespread interest and enthusiasm in the scientific community to explore their potential for the development of 188W/188Re generators. Characteristics such as high surface area, high specificity, fluid permeability, high chemical reactivity and the ability to interact with different chemical species of nanoparticles can be successfully exploited to circumvent many of the limitations pose by adsorbents based on bulk materials such as alumina.
In order to take advantage of the potential of nanomaterials as a new class of high capacity adsorbents which represent an interface between chemistry and column chromatography separation technique, captivating advances in radionuclide generators have been uncovered.93–107 Among the nanomaterials exploited for the development of 188W/188Re generators, transition metals oxides have received maximum attention.93–95,104–107 In this premise, nanostructured oxides of Ti, Zr and Al have been profusely explored by developing 9.25 GBq (250 mCi) 188W/188Re generators with performances evaluation over a period of 6 months, which is normally the shelf-life of such generators. The practical sorption capacities of all these adsorbents were reported to be much higher than the maximum achievable adsorption capacity of bulk alumina. A comparative evaluation of the sorption capacity of the nanomaterial based adsorbents for the preparation of 188W/188Re generators is provided in Table 3.
All three nanocrystalline metal oxides are amenable for preparation of clinical-scale generators using 188W obtained from high flux reactors. For example, assuming the specific activity of ∼150 GBq g−1 from high flux reactors, a 2 g column of nanocrystalline titania or zirconia could retain up to 200 mg of W corresponding to ∼90 GBq of 188W (∼2.4 Ci). This would provide a scope of preparing 37 GBq (1 Ci) generator using 1–2 g of nanocrystalline titania or zirconia. On the other hand, use of 2 g nanocrystalline alumina possessing ∼3 times higher sorption capacity would facilitate the preparation of 270 GBq (7.3 Ci) generator. It is pertinent to point out that unlike the conventional alumina based systems, 188Re obtained from these generators is of adequate radioactive concentration, requisite purity and could directly be used for the preparation of radiopharmaceuticals without PEC. The prospect of developing chromatographic 188W/188Re generators using 188W obtained from neutron irradiation of enriched (96%) 186W in medium flux (∼1014 n per cm2 per s) research reactors is worthy of consideration. It is also interesting to note that irradiation of enriched (∼96%) 186W targets for a time period of ∼6 months in medium neutron flux (∼1014 n per cm2 per s) reactors83 would result in the production of 188W of specific activity ∼6.7 GBq g−1 which could be used to produce 11.1 GBq (300 mCi) column chromatographic 188W/188Re generators using 6 g of nanocrystalline alumina.
It is clear that the development and evaluation of nanomaterial based radionuclide generator is still in its infancy, and must grow more in terms of productivity and utility. Recent advances in the design and preparation of nanomaterials confirms that numerous varieties can currently be synthesized through different synthetic routes. Having firmly established its importance for development of 188W/188Re generators, the exploration of exotic nanomaterial based adsorbents is expected to grow and excitement involved in the development of nanomaterial based 188W/188Re generators will continue to increase. With continuous research efforts, we have every reason to believe that potential steps forward will be made during the coming decade.
3.13 Solvent extraction
Solvent extraction is a classical technology used for radioisotope separations and in this regard refers to the process in which selective extraction of 188Re takes place from a liquid mixture with a solvent based on the principle of partition between two phases. This process essentially relies on the differences in the solubility of 188Re in two immiscible liquid phases. The selectivity for 188Re depends on the choice of the solvent/extractant, distribution ratio (D value) for 188Re and on the nature of the aqueous phase matrix (pH, ionic strength, etc.). Ideally the solvent/extractant should have a D value for 188Re and negligible for 188W, immiscible in aqueous phase, less viscous, nontoxic and have a significant difference in the density than that of water for simple phase separation. This method offers many advantages and offer the scope of using low specific activity 188W produced in medium flux reactors. A rapid separation procedure owing to the rapid attainment of equilibrium and this technique lends itself to a very selective separation of 188Re in terms of radionuclidic, radiochemical and chemical purity that is usually better than column chromatographic technique. These systems are usually sensitive for separation at micro- and macro level applications and therefore provide the flexibility to scale up or down to an operation level in response to requirements. A high RAC of 188Re is attainable and this technology also provides the possibility for repetition as many times as required to ensure satisfactory utilization. These systems are also amenable for automation similar to the automated system for 99mTc solvent separation from 99Mo which has been recently described.108,109Similar to all other systems some concerns have been raised on use of this technique which include the use of an intricate and bulky apparatus and the use of this extraction process is labor intensive, tedious and time consuming. The system also requires highly skilled operating and maintenance personnel, for handling the flammable and toxic organic solvents/extractants which have limited radiation stability. This is a major deterrent owing to the requirement of high degree of robustness of the operational systems for addressing safety issues.
Although the solvent extraction 188W/188Re generators based on methyl ethyl ketone (MEK) have not yet been as extensively studied as those described for the 99Mo/99mTc generator systems, some reports are available.110–112 The V. G. Khlopin Radium Institute in Russia has developed a 188Re extraction generator112 for medical purposes. Tungsten-188 used in this study was obtained by the irradiation of tungsten oxide of natural isotope composition in reactor with a mean neutron flux (1.0–1.4 × 1014 n per cm2 per s). The distribution (D) value of 188Re attains the values of 22–27 where as those of 188W were below 0.01, depending on solution compositions. While much lower than in the case of 99mTc, the D values of 188Re are sufficient to achieve complete recovery of 188Re. The parent (188W) and daughter radionuclides were separated on centrifugal semicounter-current extractor in which 188Re was extracted by MEK from alkaline solution (2.5 M KOH + 2.5 M K2CO3) containing up to 200 g L−1 W. Then MEK is evaporated to dryness and residue is dissolved in isotonic solution of NaCl. The average yield was 89% and the radiochemical purity of 188Re solution was ∼97%.
Mushtaq et al. studied the solvent extraction of 188Re in MEK63 and observed that with the increase of organic phase volume, extraction of 188Re was enhanced while the mixing time of aqueous and organic phases did not show any significant effect on the extractability of 188Re in the organic phase. In these studies it was possible to extract ∼80% of 188Re at a volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 for aqueous and organic phases. By evaporation/distillation of methyl ethyl ketone, 188Re was concentrated and reconstituted in the desired volume of physiological saline.
2 for aqueous and organic phases. By evaporation/distillation of methyl ethyl ketone, 188Re was concentrated and reconstituted in the desired volume of physiological saline.
While the solvent extraction of 188Re is an attractive method of choice for realizing the scope of using low specific activity 188W, the shortcomings of this technique limits its applicability. However, infusion of automation and better understanding of the process chemistry have not only offered a “new lease on life” to solvent extraction technology but has also made it a serious competitor of other separation technologies currently in use for fabrication of 188W/188Re generators. A facility for commercial production of 188Re using this concept has commenced operation at the Khlopin Radium Institute, St Petersburg, Russia, where it is currently used for the production of 188Re of high RAC and very low content of inorganic contaminants using 185 GBq (5 Ci) of 188W for distribution to hospitals.112 A flow chart of this process is shown in Scheme 3. In all likelihood, the prospect of using such an automated solvent extraction system in a central radiopharmacy setup appears promising.
 | ||
| Scheme 3 Flow sheet used at Khlopin's Radium Institute, St Petersburg, Russia, for the solvent extraction of 188Re. | ||
3.14 Thermochromatography
Thermochromatography (TC) is a high temperature separation process performed in a column in which volatilized species are transported along the length over which a temperature gradient is established. The temperature gradient is the specific and distinct feature of this technique. Both 188W and 188Re are deposited on the column walls at different distance depending on their temperature zones. This technology offers the scope of utilizing low-specific activity 188W. Since it is a physical process, the need for the chemical processing of neutron irradiated target is obviated.112 The process is capable of being scaled up from small batch to large batch sizes to match demand and provides 188Re of acceptable purity which remain unaltered on repeated extraction. In addition, repeated separation can be performed using the same set up over an extended period of time and provides the scope of recycling of target materials without any chemical treatment.However, the thermochromatography separation technique also has weaknesses which limit its utility. Highly specialized and complicated equipment is required, which necessitates regular or preventive maintenance. Because the goal is to perform the separation week after week all through the year, it is essential to have a very high degree of robustness of the process equipment. These complex and elaborate radiochemical separation procedures also require a skilled workforce, in addition to additional safety measures to preclude the probability and consequences of radioactive vapor release. There is also a need to perform high temperature operations, which are typically several hundred degrees centigrade on a very regular and sustainable basis. The separation efficiency of only up to 50% is attainable and the use of an open system necessitates use of a terminal sterilization of distilled 188Re prior to clinical application.
The elegant research reported for the thermochromatography concept, coupled with numerous technological innovations led to the development of a number of strategies in an attempt to separate 188Re in a chemical form amenable for the preparation of radiopharmaceuticals.113–116 Schematic representation of the thermochromatography process of 188Re separation is depicted in Fig. 8. Despite impressive progress, the promise to develop a viable clinical scale 188W/188Re generator amenable for use in a hospital radiopharmacy has not been fulfilled, and there are still many challenges and obstacles related to the sustained operation of such a system on a reliable and continuous basis.
The unfavorable economics, poor 188Re yield, complexity of the thermochromatography process and enforcement of safety measures to preclude the radioactive vapor release, have emerged as major disincentives which limit the utility of this method for laboratory research. The volume of 188Re production made possible by this route would be expected to be limited. While an outlook of the thermochromatography concept obviously hold promise and offers many stupendous advantages, the developments over the last two decades, however, have shown that it has not been easy to take full advantage of the early promises. Considerable R&D and large resources are required for its transition from the laboratory research to robust technology platform both from operational viewpoint as well as capability to provide 188Re of required quality in a seamless manner. In order to realize such an objective, evolutional progress and radical technological breakthrough are warranted. There is no doubt that in the near future, this will remain as the primary focus of many researchers.
3.15 Electrochemical separation
Electrochemical separation exploits the differences in the standard reduction potential of metal ions to separate metal ions of interest under the influence of an applied potential.117–125 This separation technique is expected to be poised to serve as a springboard to stimulate new breakthroughs and bring evolutional progress in 188W/188Re generator technology. In this process, the potential of the working electrode is maintained constant (or within a narrow range) by regulation of the voltage applied in such a way to permit the quantitative deposition (by reduction) of 188Re on an electrode surface, from a suitable electrolyte solution containing the 188W/188Re equilibrium mixture.125 A schematic diagram of the electrochemical set up used for the separation of 188Re is shown in Fig. 9.The advantages of this separation strategy include offering the scope of utilizing 188W of any specific activity since the electrode selectively deposits NCA 188Re. The process relies on oxidation–reduction reactions and is consistent with the principles of ‘green chemistry’, since electrons bring about 188Re separation without the use of external chemical reagents. The recovery of the valuable enriched 186W target after electrolysis is quantitative, which provide the prospect of target recycling and reduced costs. Since the capacity is not limited by the amount of adsorbent or extractants, it offers flexibility to scale the level of operation up or down as per demand and supply. The process not only ensures high radioactive concentration of 188Re but also high radionuclidic and chemical purity. Concerns regarding the radiolytic damage are precluded owing to the selective electrodeposition of 188Re on a metallic electrode. This process also generates minimum radioactive waste. The electrochemical generator has a long shelf life compared to other generators, with periodic addition/replacement of 188W. Separation efficiency as well as purity 188Re of remains unchanged on repeated separation and the system is amenable for automation. Although the electrochemical technique is an attractive separation method, it also has limitations and requires skilled manpower well versed in both electrochemistry and radiochemistry. The strict adherences to operating protocols are essential owing to the sensitive nature of electrochemical process.
A fully automated electrochemical 188W/188Re generator is an achievable objective since similar technology has already been developed for the 90Sr/90Y generator,117,118,126 and could be easily adopted. The important details available from the automated 90Sr/90Y generator, as well as its technical know-how, would be of considerable value for other producers and countries planning to pursue the development of automated electrochemical 188W/188Re generator. It is envisaged that the electrochemical 188W/188Re generator option would serve as a good complement in ensuring the availability of 188Re in countries with local reactor production capability to produce low to medium specific activity 188W, even as alumina column chromatographic generators based on the ORNL technology continue to remain as the main source of 188Re for most applications.
4. Transition of separation technology for the 188W/188Re generator
Advancement in separation science is the critical driver for innovation in 188W/188Re generator technology. Over the years, incremental development of existing 188W/188Re generator technologies and the creation of new technologies have proceeded in parallel at a remarkable pace. Necessities tend to spawn inventions and each change either in existing separation technology or emerging separation technology is annexed with the need of improvement and expansion of the scope as well as utility of 188W/188Re generators. As is always the case, any emerging separation technology must be sufficiently attractive and technically trustworthy to displace an existing well established technology. It is an ongoing process whereby opportunity needs to be prioritized and the effectiveness required to be assured. The advancement of separation technologies described in this paper falls in to four major categories:• Incremental change: this is primarily advances taking place within a proven separation technique and is often seen as incremental change. The post elution concentration, use of high capacity as well as nanomaterial-based adsorbent and MSIG system are included in this category.
• Paradigm change: it represents a transformational change through paradigm shifts. The change brought by the paradigm related to some form of radical innovation which transform into a new technology. The gel and electrochemical 188W/188Re generator fall in this category.
• Interruptive change: this results from the interruption of a technological pathway. The thermochromatography 188W/188Re generator concept is included in this category.
• Interdependency on other technologies: this results when a dormant technology allows a new development pathway with the help of other forces. An example in this category includes the solvent extraction 188W/188Re generator which has been resurrected with the infusion of automation.
Separation technologies which are attractive for a particular application are required to be identified, scaled up and transformed into 188W/188Re generator prototype through engineering. The steps required for such separation technology transition begins with laboratory research activities, which not only ensure greater understanding of the separation process but also reduces risk of future scaling issues. The path of transition encompasses but will not be limited to the following a diligent and rigorous laboratory research to understand the process parameters. Process development must be scaled to ensure that the 188W/188Re generator design is in line with specifications. Also, process parameters must be identified that are required to be controlled to ensure a stable and robust 188W/188Re generator system, and their optimization is necessary to ensure that process variances are managed properly to meet consistent efficiency, performance and productivity. Finally, continuous refinement of the process is required in line with the expectations.
While transition of separation processes to 188W/188Re generator is a long-term association between developers and end-users, effective collaboration among all stakeholders is the key to drive development, implementation and acceptance. Successful transition of separation technology from laboratory research to 188W/188Re generator will include flexibility – with a willingness to take risks – and open communication without regard to hierarchy. A sense of responsibility that replaces authority and a commitment to success that goes beyond functional roles will also be necessary. The separation technologies described in this paper are not necessarily competitive with one another, but instead, have progressed on different parallel tracks with a focus on developing the most robust and useful 188W/188Re generator technology.
5. Regulatory issues of 188W/188Re generator for clinical use
No discussion concerning 188W/188Re generators is complete without inclusion of a discussion of the regulatory issues required for approval for human use. The regulatory system is the key factor in the development of new 188W/188Re generators and thus in innovation. While development of new technologies for use with the 188W/188Re generator are crucial, their development and the possibility of clinical use can be compromised by a number of essentially non-technical factors, such as the complexity of legislation and regulations. Despite the encouraging prospects and the favorable developments, there is still quite a long path before any new separation technology can be incorporated into a new 188W/188Re generator prototype for use in daily nuclear medicine routine. Success in this endeavor depends on operational excellence in all phases of the production chain from early research up to the regulatory process and the market.Rhenium-188 obtained from 188W/188Re generators is considered as an active pharmaceutical ingredient (API) since it is used as a starting material for the preparation of radiopharmaceuticals for human use.127 Therefore, 188W/188Re generators preparation and use of 188Re are regulated by a number of national and international directives, regulations and rules. In addition to the design specifications, installation, verification, and maintenance protocols, the current Good Manufacturing Practice (cGMP) regulations are the minimum set of requirements for 188W/188Re generator to be complied for clinical use. Enforcement of cGMP is designed to preclude patients at risk due to inadequate safety and quality, and to enhance consistency in the application of the regulatory requirements. With multiple elutions of 188Re from generators and the preparation of multiple 188Re radiopharmaceuticals per day and combined with the trend towards higher patient dosages, radiation safety issues are also major concerns. With a view to ensure regulatory compliance, it is essential to have a comprehensively designed and correctly implemented quality management system that incorporates cGMP, quality assurance and control, lifecycle and risk management as appropriate, as well as activities necessary to ensure confidence that the 188Re obtained from the 188W/188Re generator will meet its intended pharmacopeia specifications for quality and purity.
The U.S. Food and Drug Administration (FDA) procedures for the preparation and processing of 186(188)W targets and generator fabrication and performance evaluation and acceptance testing must be documented in a Drug Master File (DMF). One DMF for the manufacture of a non-sterile 188W/188Re generator as an API had already been filed by ORNL. The approved regulations describe production of radionuclides used as an API according to cGMP, and are outlined in the Code of Federal Regulations. Basic requirements of cGMP include clearly defined and appropriately controlled 188W/188Re generator manufacturing processes which are required to ensure consistency and compliance with approved specifications. Independent quality unit(s) should be in place in the form of separate quality assurance (QA) and quality control (QC) units or a single individual or group, depending upon the size and structure of the organization to undertake both QA and QC responsibilities. Importantly, critical steps of the 188W/188Re generator fabrication processes and significant changes to the process must be validated. It is also essential to have an adequate number of well-trained qualified personnel, operators, adequate premises and facilities, suitable equipment and utilities, approved procedures and instructions, and appropriate storage and transport facilities. In general, 188W/188Re generator manufacturing operations should also be performed under the responsibility of personnel with appropriate competence in radiation protection. Instructions and procedures of 188W/188Re generator manufacturing processes must be adequately documented in clear and unambiguous language. All quality related activities as well as deviation from established procedures should be systematically documented and critical deviations are to be investigated and documented properly. Finally, records of fabrication, packaging, and distribution to hospital nuclear medicine department, radiopharmacy or licensed healthcare professional, must be retained in a comprehensible and accessible form.
Although the 188W/188Re generators can be cGMP manufactured as an API and need not be sterile/non-pyrogenic products, their introduction and subsequent radiopharmacy use for preparation of agents for human use must comply with “Guidelines on Good Radiopharmacy Practice” requirements, and “Guidance on current Good Radiopharmacy Practice (cGRPP)”. The 188W/188Re generators are produced in compliance with cGMP to ensure that under the specified operating conditions the generator is able to provide 188Re in the desired quantity and quality specified. A dossier on each 188W/188Re generator produced is required to be submitted as a pre-requisite before entry into distribution chain. Adequate measures should also be in place to ensure that 188W/188Re generators are stored and handled in such a way that the required quality of 188Re obtained from the generator can be assured throughout their shelf-life. Rhenium-188 used in radiolabeling is required to be analyzed according to a monograph of a pharmacopoeia – when available – and must comply with the requirements of the pharmacopoeia. All analyses have to be performed in accordance with national regulations prior to patient use with the exception of tests for sterility and pyrogens. While the tests for sterility and apyrogenicity remain standard quality testing procedures, this is required to be carried out in batch or composite samples collected for random testing of “processing methods”. Materials used for the preparation of 188Re radiopharmaceutical as excipients (solvents, buffers, stabilizers, additives, antimicrobial agents, etc.) must be of pharmacopoeia quality (as indicated on the label), or be accompanied by a certificate of analysis, or be analyzed using validated methods and in accordance with national regulations.
In addition to meeting pharmaceutical GMP regulations, manufacturers in the U.S. undertaking regular production of 188W/188Re generator must generally be licensed by a Nuclear Regulatory Authority (NRA) (i.e. DOE facilities like ORNL are exempt from NRC regulations). In this context, it is mandatory for the manufacturer to demonstrate that its facility used for 188W/188Re generator production is adequate to protect health and minimize danger to life or property. Additionally, the manufacturer is required to be qualified to use radioactive material (188W/188Re solution), established a radiation protection program, as well as controls and procedures for management, record keeping, accounting, and use of radioactive materials.
As part of the cGMP quality program, researchers at ORNL implemented and filed documentation of the manufacturing processes of 188W/188Re generator as a Drug Master File with the US FDA. The Drug Master File document contains details of all production, processing and quality steps, including chemistry, stability, purity, packaging, and other pertinent information. The implementation cGMP program at ORNL is regarded as an important step forward in providing 188W/188Re generator, based on their groundbreaking work, as a non-sterile Active Pharmaceutical Ingredient (API) for broader clinical use.128 The European Medicines Agency (EMEA) approved 188W/188Re generators have also been available from IRE, Fleurus, Belgium, POLATOM, Otwock, Poland and ITG, Garching, Germany.
In contrast to the transport of conventional pharmaceutical ingredients, the shipment of 188W/188Re generators to the end user from the manufacturer is regulated by national and international regulatory authorities adhering to the safe transport of radioactive material procedure. Each shipment of 188W/188Re generators to a hospital-based nuclear medicine department, radiopharmacy or licensed healthcare professional must be accompanied with shipping documents that identify the radionuclide, physical and chemical form of the material, and the activity contained of 188W/188Re generator. The manufacturer is required to retain the shipping papers for each generator for three years and provided to authorized government or regulatory authority officials on request.
6. Central radiopharmacy concept and its impact on the use of 188W/188Re generators
The existing practice of using 188W/188Re generators in nuclear medicine centers in a hospital-based radiopharmacy (HRPh) is expected to converge, making it likely that future supplies will take place through centralized radiopharmacies (CRPh) set up to achieve the compliance with cGMP.129–131 Although the central radiopharmacy concept is not widely practiced on an international basis, there are about 135 such radiopharmacies currently operating in the US, which provides a model for further expansion to other regions/countries of this important concept to reduce costs. In this regard, 188Re obtained from a 188W/188Re generator, unlike normal medicines, must undergo total quality control assessment conditions overseen by a state registered pharmacist or “authorized person/qualified person” before undertaking preparation of radiopharmaceuticals to insure patient safety. In the light of the perceived need to address these requirements, the prospect of distributing 188Re through centralized radiopharmacies is enticing as both from a legal and quality assurance perspective as the responsibility for the quality of the 188Re resides in the hands of the centralized radiopharmacy. Major benefits of using a centralized radiopharmacy service include the prospect for efficient and cost effective utilization of 188W/188Re generators as it ensures recovery of 188Re from available 188W/188Re generators. In addition to simplifying regulatory and practice-based paperwork, the CRPh significantly reduces skilled manpower requirements for the hospital-based generator operation as well as preparation of 188Re-labeled radiopharmaceuticals. The implementation of GMP's is required during the elution of 188Re from the generator and post elution processing operation and efficient quality control of 188Re by involvement of trained individuals available at the CRPh. These procedures enhance the ability to dispense patient specific unit doses of 188Re activity or radiopharmaceuticals of the required quality where procurement of a 188W/188Re generator may be cost ineffective or prohibitive. In addition to reduction of radiation exposure to personal, the probability of contamination is also decreased, which can be minimized owing to the availability of a properly designed and constructed CRPh facility. Other requirements include establishment of an efficient storage system for management of radioactive waste for returning waste to a central site. These steps provide the scope for establishing a 188Re radiopharmacy research and development program and a training site for nuclear medicine technologists, physicians, pharmacists and other staff involved in the preparation and use of 188Re-labeled radiopharmaceuticals.While implementation of CRPhs is driven more by needs in meeting regulatory demands than by commercial interests alone, one of the major benefits for the CRPh is the availability and centralization of expertise which may not be feasible or justifiable in a decentralized system, such as a HRPh.132 With the relative advantages of a CRPh compared to a HRPh, the discussion has emerged whether centralized or non-centralized availability of 188Re from 188W/188Re generators is preferable. The choice will be made by those individuals who make decisions, but a number of considerations must be taken into account. While the costs of 188Re could be reduced by centralized production by more efficient use of 188W/188Re generators, the potential loss of radioactivity by decay during transportation and impact of transportation (timing, traffic, costs) should be evaluated. The availability of 188Re from the CRPh may be at the expense of HRPh where significant expertise on 188Re tracer development and innovation are available. It remains to be seen if HRPh researchers would continue the development of 188Re tracers if demand for 188Re is met through CRPh efforts. Arguments to continue HRPh include the importance of preservation of expertise for 188Re tracer technology and infrastructure. An argument to discontinue HRPh is the relocation of research and development work. The capabilities and services of a CRPh must be available and shared by multiple institutions, if not all, hospitals in a given region to be cost effective and economically sustainable.
In the future, where possible, it is anticipated that institutions and investigators who require 188Re will obtain from the CRPh for an increasing portion of their 188Re needs, since these facilities are able to meet demands in a safe, timely, and cost efficient manner. The installation and greater efficient use of 188W/188Re generators in CRPh facilities would be expected to drastically reduce the required number of required 188W/188Re generators. In general, the generator activity levels required at a CRPh are significantly higher than that used in HRPh. The 188W/188Re generator is expected to undergo a paradigmatic shift from the present designs owing to altered user profiles. New 188W/188Re generators must be carefully configured and managed appropriately to provide 188Re of requisite purity and quantities. In view of this, it is of utmost importance to assure access to appropriate separation technologies that will provide clinical grade 188Re. In this premise, proven separation technologies are expected to be quickly restructured and at the same time, emerging technologies can be nurtured in an appropriate manner to respond to need of CRPh.
7. Infusion of automation in 188W/188Re generator to expand its scope and utility
In light of the explicit need to handle high activity level 188W/188Re generators, the prospect for further development, optimization and use of automation is expected not only to expand the scope and utility for use of this generator, but would also represent a stepping stone towards achieving cGRPP compliance. In this context both semi-automated and fully automated system 188W/188Re generator should be given further serious consideration. One advantage in adopting semi-automated 188W/188Re generator system is that it will be better able to meet regulatory needs and introduced to the market. But if the semi-automated 188W/188Re generator is used to satisfy regulatory demands, after the 188Re obtained from the generator is approved, conversion of the semi-automated to automated unit will be strategic and can delay the requirement to invest more capital for an automated 188W/188Re generator. While the use of semi-automated 188W/188Re generators, whereby the operator still has to interfere in the process of isolating generator produced radionuclide has tangible benefits, availability and use of fully automated system using a computer controlled interface is very desirable. The progressive fusion of existing generator technologies with automation would not only ensure a sustained growth but also empower future developments. The advantages of using automated 188W/188Re generator include the scope for optimum 188W/188Re generator utilization. Use of a fully automated 188W/188Re generator without user intervention leads to reduction of 188Re production time, significant reduction in radiation exposure to the operator and elimination of human error, in addition, it also offers consistent performance of the generator system in terms of reproducibility of 188Re purity and yield. Automation also offers low operational variability as regards to elution of 188Re is concerned and an automated 188W/188Re generator guarantees robustness. In addition it provides the scope of maintaining a data base of steps performed, including documentation of all process parameters and functions during 188Re elution. An automated 188W/188Re generator can guarantee better control of sterility and apyrogenicity of 188Re because of minimal human intervention. Standardized automated 188W/188Re generators are easy to transfer to hospitals or radiopharmacy settings without compromising performance.Taking advantage of automation, new advances in 188W/188Re generator technology can be identified. While automation holds promise and offers numerous advantages and has the potential to offer use of separation technologies which may have been abandoned, it is associated with the challenge of re-configuring the generator technology that requires integration of several separation steps while maintaining full automation. This strategy must be hastened further and nurtured in ways that respond to changing times.
8. Conclusions and future prospects
We have reviewed the use of various separation technologies which exploit differences in chemical properties of 188W and 188Re. A review of these separation techniques indicates that this field is rapidly evolving. Almost every advance in 188W/188Re separation technologies has been depicted as a breakthrough, and the list of emerging technologies steadily increases. While a variety of emerging separation technologies have the potential to be applied for development of new 188W/188Re generator prototypes, it is important to focus on separation technologies which represent the most promising technologies.Any discussion of 188W/188Re generators begins with a description of the column chromatographic technique. Without doubt, this simple yet elegant technique has offered pharmaceutical grade 188Re and allowed the clinical evaluation of new 188Re agents. The current most widely used alumina-based chromatographic 188W/188Re generator system has resulted from years of technological investment. Recent changes have represented incremental advances. Widespread introduction of this column chromatographic 188W/188Re generator continues to be handicapped by the limited W binding capacity of the alumina adsorbent. For this reason, this system relies on the use of the highest 188W specific activity which is currently only available from two reactor sources (SM3 Reactor, RIAR, Dimitrovgrad, Russia and HFIR of ORNL, TN, US). The limited availability and high costs of the 188W/188Re generators continues to negatively affect 188Re-based therapy as well as research activities on 188Re based radiopharmaceuticals.
In order to mitigate this contradictory association of good generator performance but limited 188W specific activity, and to expand the scope for use of these generators, a paradigm shift is required within the framework of column chromatography. In this context, the use of the MSIG concept described in this review represent a pragmatic approach which may pave the way for broad use of the low specific activity 188W available from many reactors worldwide, that would never have been realized using the established column chromatography technique. Use of nanomaterial-based adsorbents has proved to be an intuitive proposition which offers the flexibility of using a range of 188W specific activities. Although use of nanomaterial-based adsorbents for the development of column chromatographic 188W/188Re generators is in its infancy, this technology may have good prospects. While innovation in the column chromatographic techniques has been demonstrated, its impact for improvements in 188W/188Re generator technology is only beginning to be realized.
The continuing discussion about use of the column chromatographic technique and the roles of alternative separation strategies for separation of 188Re from 188W and specific activity of 188W, have not been widely described. This must be further discussed not only because a greater range of 188W/188Re generator options will be required, but also for the adaptability to use lower specific activity 188W. Recent advances in separation technology involve a variety of technologies, which could lead to substantial improvements. In order to realize the advantages of emerging separation technologies, it is imperative to evaluate innovative approaches. In this context, the scope of using the electrochemical separation technique for the 188W/188Re generator should be further evaluated. This technology also provides scope for use of lower specific activity 188W and is expected to overcome many limitations encountered with traditional column chromatography methodologies. Recently reported and continuing research on electrochemical 188W/188Re generator concept, coupled with technological innovations, may thus open the door for future possibilities.
With increasing clinical use of 188Re-labeled radiopharmaceuticals and preference toward unit dose requirements, the prospect of providing 188Re from 188W/188Re generators available from CRPh is appealing. The CRPh had been an innovative concept and has now witnessed prolific growth to provide unit doses of many radiopharmaceutical agents. Such systems could also provide the required 188Re doses for therapy and at the same time further stimulate the clinical use of 188Re-radiopharmaceuticals. Harnessing of the 88W/188Re generator technology in conjunction with its convergence with CRPh may be expected to be poised to disrupt the 188W/188Re generators technology status quo, alter its operation, and rearrange value pools and services. In the final outcome, the ability to provide 188Re by the CRPh system is dictated by the availability of high activity 188W/188Re generators. There is no apparent barrier for the use of 188W/188Re generators based on non-conventional separation technologies in CRPh's because of the availability of highly qualified manpower. While 188W/188Re generators based on alternative separation techniques are not yet widely available, the potential of the MSIG approach, nanomaterial-based adsorbent and electrochemical concepts are very attractive given current trends in the evolution of the CRPh concept. Such approaches are realistic, would be expected to be implementable in the CRPh system and are capable of providing radiopharmaceutical grade 188Re for radionuclide therapy. While the development and expected use of 188W/188Re generators based on alternative separation techniques is a worthwhile goal, quality assurance systems must be comprehensively and correctly implemented to ensure that quality and safety of 188Re is adequate for intended use. Although considerable experience has been accumulated over the years by CRPh facilities, the full potential of CRPh's has not yet been fully exploited.
Despite the established progress in separation technology development, these technologies have not yet been translated for the development of 188W/188Re generator prototypes which have been used for clinical applications, a situation largely attributed to technical, regulatory and marketing challenges. While many 188W/188Re generator technologies have demonstrated utility, there are persistent regulatory challenges and uncertainties impeding their prospects for clinical translation and commercialization. In addition to the current high costs of reactor-produced 188W, other specific issues relate to the regulatory cost burden and timelines involved in the transition are largely due to GMP requirements. The situation is compounded by uncertainties associated with the lack of harmonization in how regulations are implemented and interpreted on international basis. In order to surmount regulatory barriers, it is essential to have a constructive alliance between regulatory bodies, stakeholders and researchers to seek mutually acceptable and appropriate levels of solution to enable innovative 188W/188Re generators to be delivered to the healthcare sector without compromising safety, quality and efficacy.
Combining advances in separation science with recent breakthroughs in automation would propel 188W/188Re generator technology forward. The goal is to provide 188Re in a seamless manner amenable for a variety of clinical applications. The great potential of automation provides countless advantages and technological breakthroughs. This technology not only represents an important driver to redefine the next generation 188W/188Re generator technologies, but will also provide technological solutions for the resurrection of some of the multistep elaborate separation technologies such as solvent extraction of 188Re using MEK which had been previously considered unproductive and abandoned. While automation of 188W/188Re generator technology is important, it is also associated with substantial cost implications. For this reason, it is therefore worthwhile to develop a logical framework approach that addresses the broad spectrum of technical challenges.
As technology transformation is pursued, it is of utmost importance to retain the existing, proven, still-important 188W/188Re technology options with a view to maintain a balance with continuity. Indeed, the goal is to make sustained, affordable, and achievable improvements in the existing 188W/188Re generator technologies that could be constantly sought, preserved, and re-ratified. While the use of low specific activity 188W using alternative separation techniques to develop 188W/188Re generators is a new paradigm to expand scope and utility, a constant and reliable supply of 188W of the required quality in the desired quantities must be assured. Clearly, success in this direction demands a detailed restructuring of irradiation schedules at the existing operating moderate flux research reactors. If such proposals are adopted, one could expect in the near term a large variety of 188W/188Re generators capable of providing clinical grade 188Re using low specific activity 188W.
While the 188W/188Re generator technology has passed many milestones, it is essential to work hand-in-hand with university, national laboratories and private sector partners to harness innovation in separation science, advancement in technology as well as automation, and partnerships to push 188W/188Re generator technology forward. This would lay the foundation for the 188W/188Re generator of the future. With continuous efforts in this direction, we have every reason to believe that major step forward will be taken in the coming years to develop innovative, cost-efficient and sustainable 188W/188Re generator prototypes that can address the increasing needs for cost effective and readily available therapeutic radioisotopes in a comprehensive manner. Finally, all stakeholders in the nuclear medicine community including research scientists, clinicians, radiopharmacists, hospitals and industry require sharing a common platform to facilitate exploration of new 188W/188Re generator technology through innovations; identify the technologies that would best suit needs, and determine how to work within the required regulatory framework.
Abbreviations
| ABEC | Aqueous biphasic extraction column |
| API | Active pharmaceutical ingredient |
| α | Separation factor |
| β− | Beta particle |
| BPI | Bulk pharmaceutical ingredient |
| BRIT | Board of Radiation and Isotope Technology (India) |
| CRPh | Centralized radiopharmacy |
| cGRPP | Guidance on current good radiopharmacy practice |
| Ci | Curie |
| D | Distribution |
| DEAE | Diethyl amino ethyl cellulose |
| DM | Demineralized |
| DMF | Drug master file |
| DMSA | Dimercaptosuccinic acid |
| DOE | Department of Energy |
| E(βmax) | Maximum beta energy |
| EMEA | European Medicines Agency |
| FDA | Food and Drug Administration |
| γ | Gamma emission |
| GBq | Gigi becquerel |
| GC | Guard column |
| cGMP | Current good manufacturing practice |
| GMP | Good manufacturing practice |
| HCC | Hepatocellular carcinoma |
| HEDP | Hydroxyethylidendiphosphonic acid |
| HFIR | High flux isotope reactor |
| HRPh | Hospital based radiopharmacy |
| In vitro | Within an artificial environment |
| In vivo | Within the living |
| IAEA | International Atomic Energy Agency (Vienna) |
| IVRT | Intravascular radionuclide therapy |
| KD | Distribution coefficient |
| keV | Kilo electron volts |
| LSA | Low specific activity |
| mCi | Milli curie |
| MEK | Methyl ethyl ketone |
| MSIG | Multicolumn selectivity inversion |
| NCA | No carrier added |
| NRC | Nuclear Regulatory Commission |
| ORNL | Oak Ridge National Laboratory (TN) |
| PEC | Post elution concentration |
| PSC | Primary separation column |
| PTC | Polymeric titanium oxychloride |
| PZC | Polyzirconium compound |
| QA | Quality assurance |
| QC | Quality control |
| RAC | Radioactive concentration |
| 188Re | Rhenium-188 |
| R&D | Research and development |
| RIAR | Reactor Institute for Atomic Research |
| RIT | Radioimmunotherapy |
| RNT | Radionuclide therapy |
| SCK·CEN | Studiecentrum voor kernenergie, centre d'Étude de l'énergie nucléaire |
| Sr | Strontium |
| TC | Thermochromatography |
| U | Uranium |
| 188W | Tungsten-188 |
| Y | Yttrium |
References
- F. F. Knapp Jr, M. R. A. Pillai, J. A. Osso Jr and A. Dash, J. Radioanal. Nucl. Chem., 2014, 303, 1053 CrossRef PubMed.
- F. F. Knapp Jr and P. Baum, Curr. Radiopharm., 2012, 6, 175–177 CrossRef.
- R. M. Lambrecht, Radiochim. Acta, 1983, 34, 9 CAS.
- F. F. Knapp Jr and S. Mirzadeh, Eur. J. Nucl. Med., 1994, 21, 1151 CrossRef CAS.
- S. Mirzadeh and F. F. Knapp Jr, J. Radioanal. Nucl. Chem., 1996, 203, 471 CrossRef CAS.
- Radionuclide Generators: New Systems for Nuclear Medicine Applications, ed. F. F. Knapp Jr and T. A. Butler, ACS Symposium Series, American Chemical Society, United States, 1984, vol. 241 Search PubMed.
- J. Osso Jr and F. F. Knapp Jr, Principles and Operation of Radionuclide Generators, in Sampson's Textbook of Radiopharmacy, ed. T. Anthony, Pharmaceutical Press, London, 2011, p. 339 Search PubMed.
- F. Roesch and F. F. Knapp Jr, Radionuclide Generators, in Radiochemistry and radiopharmaceutical chemistry in life sciences: Handbook of Nuclear Chemistry, ed. A. Vertes, S. Nagy and Z. Klenscar, Kluwer Academic Publisher, The Netherlands, 2003, p. 81 Search PubMed.
- R. Chakravarty and A. Dash, Development of radionuclide generators for biomedical applications, Lambert Academic Publishing, Germany, 2013 Search PubMed.
- A. P. Callahan, D. E. Rice and F. F. Knapp Jr, NucCompact, 1989, 20, 3 CAS.
- F. F. Knapp Jr, E. C. Lisic, S. Mirzadeh and A. P. Callahan, U.S. Pat., No. 5,186,913, February 17, 1993.
- F. F. Knapp Jr, S. Mirzadeh and A. L. Beets, J. Nucl. Med., 2000, 41, 149 Search PubMed.
- F. F. Knapp Jr, A. P. Callahan, A. L. Beets, S. Mirzadeh and B.-T. Hsieh, Appl. Radiat. Isot., 1994, 45, 1123 CrossRef.
- F. F. Knapp Jr, J. H. Turner and A. K. Padhy, World J. Nucl. Med., 2004, 3, 137 Search PubMed.
- B.-T. Hsieh, A. P. Callahan, A. L. Beets, G. Ting and F. F. Knapp Jr, Appl. Radiat. Isot., 1996, 47, 23 CrossRef CAS.
- F. F. Knapp Jr, A. L. Beets, S. Guhlke, P. O. Zamora, H. Bender, H. Palmedo and H. J. Biersack, Anticancer Res., 1997, 17, 1783 CAS.
- J. M. Jeong and F. F. Knapp Jr, Semin. Nucl. Med., 2008, 38, S19 CrossRef PubMed.
- M. R. A. Pillai, A. Dash and F. F. Knapp Jr, Curr. Radiopharm., 2012, 4, 228 CrossRef.
- F. F. Knapp Jr, Cancer Biother. Radiopharm., 1998, 13, 337 CrossRef CAS.
- B. Lambert and J. M. de Klerk, Nucl. Med. Commun., 2006, 27, 223 CrossRef CAS PubMed.
- J. M. Jeong and J. K. Chung, Cancer Biother. Radiopharm., 2003, 18, 707 CrossRef CAS PubMed.
- S. Jürgens, W. A. Herrmann and F. E. Kühn, J. Organomet. Chem., 2014, 751, 83 CrossRef PubMed.
- G. Ferro-Flores and C. Arteaga de Murphy, Adv. Drug Delivery Rev., 2008, 60, 1389 CrossRef CAS PubMed.
- M. Frier, Mini-Rev. Med. Chem., 2004, 4, 61 CrossRef CAS.
- U. Abram and R. Alberto, J. Braz. Chem. Soc., 2006, 17, 1486 CrossRef CAS PubMed.
- M. Argyrou, A. Valassi, M. Andreou and M. Lyra, Int. J. Mol. Imaging, 2013, 2013, 290 Search PubMed.
- E. Deutsch, K. Libson, J. L. Vanderheyden, A. R. Ketering and R. Maxon, Nucl. Med. Biol., 1986, 13, 465 CAS.
- C. Bolzati, A. Boschi, L. Uccelli, A. Duatti, R. Franceschini and A. Piffanelli, Nucl. Med. Biol., 2000, 27, 309 CrossRef CAS.
- G. Liu and D. J. Hnatowich, Med. Chem., 2007, 7, 367 CAS.
- A. P. Callahan, S. Mirzadeh and F. F. Knapp Jr, Radioact. Radiochem., 1992, 3, 46 CAS.
- S. Mirzadeh, F. F. Knapp Jr and A. P. Callahan, Production of tungsten-188 and osmium-194 in a nuclear reactor for new clinical generators, in Nuclear Data for Science and Technology, 1992, p. 595 Search PubMed.
- F. F. Knapp Jr, A. P. Callahan, A. L. Beets and S. Mirzadeh, Appl. Radiat. Isot., 1994, 45, 1123 CrossRef.
- M. Garland, Neutronic effects of tungsten-186 double neutron capture, Ph.D. thesis, University of Maryland, 2004.
- A. Mushtaq, Appl. Radiat. Isot., 1996, 47, 727 CrossRef CAS.
- S. Mirzadeh, F. F. Knapp Jr and R. M. Lambrecht, Radiochim. Acta, 1997, 77, 99 CAS.
- F. F. Knapp Jr, S. Mirzadeh, M. Garland, B. Ponsard and R. A. Kuznetzov, Reactor production and processing of 188W. In Production of long lived parent radionuclides for generators.: 68Ge, 82Sr, 90Sr and 188W, Bibliogr. Ser., I. A. E. A., 2010, 79 Search PubMed , http://www-pub.iaea.org/MTCD/publications/PubDetails.asp?pubId=8268.
- Y. G. Toporov, Y. G. Tarasov, R. A. Kuznetsov and G. V. Goncharova, Production of W-188, Book of Abstracts, Second Russian Conf. on Radiochemistry, Dimitrovgrad, 1997, p. 265 (in Russian) Search PubMed.
- R. A. Kuznetsov, V. A Tarasov, S. I. Klimov, A. N. Pakhomov and O. V. Bubas, Production of 188W in SM high-flux reactor at SSC RF RIAR, Proceedings of the 5th international conference on isotopes, Brussels, Belgium, April 25-29, 2005, p. 109 Search PubMed.
- B. Ponsard, J. Hiltunen, P. Penttilla, H. Vera Ruiz, A. L. Beets, S. Mirzadeh and F. F. Knapp Jr, J. Radioanal. Nucl. Chem., 2003, 257, 169 CrossRef CAS.
- A. Dash, F. F. Knapp Jr and M. R. A. Pillai, RSC Adv., 2013, 3, 14890 RSC.
- R. Chakravarty and A. Dash, J. Radioanal. Nucl. Chem., 2014, 299, 741 CrossRef CAS PubMed.
- A. Dash and R. Chakravarty, RSC Adv., 2014, 4, 42779 RSC.
- E. M. Huffman, R. L. Oswalt and L. A. Williams, J. Inorg. Nucl. Chem., 1956, 3, 49 CrossRef CAS.
- J. Blachot, J. Hermet and A. Moussa, Int. J. Appl. Radiat. Isot., 1969, 20, 467 CrossRef CAS.
- R. L. Hayes and J. J. Rafter, in Research Report, Medical Division, Oak-Ridge Associated Universities, ORAU, 1965, vol. 101, p. 74 Search PubMed.
- R. L. Hayes and J. J. Rafter, J. Nucl. Med., 1966, 7, 797 Search PubMed.
- R. E. Lewis and J. S. Eldrige, J. Nucl. Med., 1966, 7, 804 Search PubMed.
- K. V. Malyshev and V. V. Smirnov, Radiokhimia, 1975, 17, 249 CAS.
- G. Ehrhardt, A. P. Ketring, T. A. Turpin, M. S. Razavi, J.-L. Vanderheyden and A. R. Fritzberg, J. Nucl. Med., 1987, 28, 656 Search PubMed.
- A. P. Callahan, D. E. Rice and F. F. Knapp Jr, J. Nucl. Med., 1987, 28, 657 Search PubMed.
- C. Klofutar, F. Krašovec and A. Kodre, J. Radioanal. Chem., 1970, 5, 3 CrossRef CAS.
- N. S. Mikheev, V. S. Popovich, I. A. Rumer and N. C. Volkova, Isotopenpraxis, 1972, 8, 248 CAS.
- N. Botros, M. El-Garhy, S. Abdulla and H. F. Aly, Isotopenpraxis, 1986, 10, 368 CrossRef.
- G. Kodina, T. Tulskaya, E. Gureev, G. Brodskaya, O. Gapurova and B. Drosdovsky, Production and Investigation of Rhenium-188 Generator, in Technetium and Rhenium in Chemistry and Nuclear Medicine 3, ed. M. Nicolini and G. Bandoli, Corina International, 1990, p. 635 Search PubMed.
- F. F. Knapp Jr, E. J. Lisic, S. Mirzadeh, A. P. Callahan, D. E. Rice and E. C. Lisic, Eur. J. Nucl. Med., 1991, 18, 538 Search PubMed.
- H. Kamioki, S. Mirzadeh, R. M. Lambrecht, F. F. Knapp Jr and K. Dadachova, Radiochim. Acta, 1994, 65, 39 CAS.
- E. C. Lisic, A. P. Callahan, S. Mirzadeh and F. F. Knapp Jr, Radioact. Radiochem., 1992, 3, 42 CAS.
- F. F. Knapp Jr, A. L. Beets, S. Mirzadeh and S. Guhlke, Use of a new tandem cation/anion exchange system with clinical scale generators provides high specific volume solutions of technetium-99m and rhenium-188, in Modern Trends in Radiopharmaceuticals for Diagnosis and Therapy, IAEA-TECDOC-1029, 1998, p. 419 Search PubMed.
- F. F. Knapp Jr, A. L. Beets, S. Mirzadeh and S. Guhlke, U.S. Pat., No. 5,729,821, issued March 17, 1998.
- S. Guhlke, J. Labelled Compd. Radiopharm., 1998, 40, 94 Search PubMed.
- S. Guhlke, A. L. Beets, K. Oetjen, S. Mirzadeh, H.-J. Biersack and F. F. Knapp Jr, J. Nucl. Med., 2000, 41, 1271 CAS.
- S. K. Sarkar, M. Venkatesh and N. Ramamoorthy, Appl. Radiat. Isot., 2009, 67, 234 CrossRef CAS PubMed.
- A. Mushtaq, T. H. Bukhari and I. U. Khan, Radiochim. Acta, 2007, 95, 535 CrossRef CAS.
- R. Chakravarty, A. Dash, M. R. A. Pillai and M. Venkatesh, Appl. Radiat. Isot., 2010, 68, 2302 CrossRef CAS PubMed.
- B. Jäckel, R. Cripps, S. Güntay and H. Bruchertseifer, Appl. Radiat. Isot., 2005, 63, 299 CrossRef PubMed.
- G. Wunderlich, H. Hartmann, M. Andreeff and J. Kotzerke, Appl. Radiat. Isot., 2008, 66, 1876 CrossRef CAS PubMed.
- E. P. Horwitz and A. H. Bond, U.S. Pat., 6,998,052, February 14, 2006.
- E. P. Horwitz and A. H. Bond, Czech J. Phys., 2003, 53(suppl. A), A713 CrossRef CAS.
- S. K. Spear, S. T. Griffin, J. G. Huddleston and R. D. Rogers, Ind. Eng. Chem. Res., 2000, 39, 3173 CrossRef CAS.
- J. E. Young and J. J. Hines, U.S. Pat., 6, 770,1 95, August 3, 2004.
- H. Bond, J. J. Hines, J. E. Young and E. P. Horwitz, U.S. Pat., 6,787,042, September 7, 2004.
- D. R. Mcalister and E. P. Horwitz, Appl. Radiat. Isot., 2009, 67, 1985 CrossRef CAS PubMed.
- T. J. Morley, M. Dodd, K. Gagnon, V. Hanemaayer, J. Wilson, S. A. McQuarrie, W. English, T. J. Ruth, F. Bénard and P. Schaffer, U.S. Pat., 6770195, 21 June, 2002.
- A. H. Bond, J. J. Hines, J. E. Young and P. E. Horwitz, U.S. Pat., 6787042, 21 June, 2002.
- RadioGenix™, NorthStar Medical Radioisotopes, LLC, http://mo99.ne.anl.gov/2014/pdfs/presentations/S3P3%20Presentaton%20Harvey.pdf.
- R. E. Boyd, Appl. Radiat. Isot., 1997, 48, 1027 CrossRef CAS.
- K. V. Malyshev and V. V. Smirnov, Radiokhimia, 1975, 17, 249 CAS.
- G. Ehrhardt, A. P. Ketring, T. A. Turpin, M. S. Razavi, J.-L. Vanderheyden and A. R. Fritzberg, J. Nucl. Med., 1987, 28, 656 Search PubMed.
- G. J. Ehrhardt, R. G. Wolfangel and E. A. Deutch, U.S.Pat., 5,382,388, Jan.17, 1995.
- G. J. Ehrhardt, U.S.Pat., 4859431 A, Jan 25, 1988.
- M. Dadachov, R. M. Lambrecht and E. Hetheringtion, J. Radioanal. Nucl. Chem., 1994, 188, 267 CrossRef CAS.
- M. S. Dadachov, V. S. Le, R. M. Lambrecht and E. Dadachova, Appl. Radiat. Isot., 2002, 57, 641 CrossRef CAS.
- M. S. Dadachov and R. M. Lambrecht, J. Radioanal. Nucl. Chem., 1995, 200, 211 CrossRef CAS.
- H. Matsuoka, K. Hashimoto, Y. Hishinuma, K. Ishikawa, H. Terunuma, K. Tatenuma and S. Uchida, J. Nucl. Radiochem. Sci., 2005, 6, 189 CrossRef CAS.
- L. V. So, C. D. Nguyen, P. Pellegrini and V. C. Bui, Sep. Sci. Technol., 2009, 44, 1074 CrossRef.
- J. A. Flores de la Torre, V. E. Badillo Almaraz, F. Monroy-Guzman and F. F. Knapp Jr, Hydroxyapatite-based 99Mo/99mTc and 188W/188Re generator systems, Proceeding of International symposium on trends in radiopharmaceuticals (ISTR-2005), International Atomic Energy Agency (IAEA), Vienna,(Austria), 2005, p. 52 Search PubMed.
- F. Monroy-Guzman, V. E. Badillo Almaraz, J. A. Flores de la Torre, J. M. Cosgrove, and F. F. Knapp Jr, Hydroxyapatite-Based Mo-99/Tc-99m and W-188/Re-188 generator systems, in Trends in Radiopharmaceuticals (ISTR-2005), Proceedings of the International Symposium, Vienna, Austria, 2007, vol. 1, pp. 333–348 Search PubMed.
- E. Iller, H. Polkowska-Motrenko, D. Wawszczak, M. Konior and J. Milczarek, Annual Report, Radioisotope Centre POLATOM, 2007, vol. 4, p. 102 Search PubMed.
- H. Iller, W. Polkowska-Motrenko, D. Łada, M. Wawszczak, K. Sypuła, M. Doner, J. Konior, J. Milczarek and Z. J. Ralis, J. Radioanal. Nucl. Chem., 2009, 281, 83 CrossRef.
- B. Lee, H. J. Im, H. Luo, E. W. Hagaman and S. Dai, Langmuir, 2005, 21, 5372 CrossRef CAS PubMed.
- B. Lee, L. L. Bao, H. J. Im, S. Dai, E. W. Hagaman and J. S. Lin, Langmuir, 2003, 19, 4246 CrossRef CAS.
- J. S. Lee, J. S. Lee, U. J. Park, K. J. Son and H. S. Han, Appl. Radiat. Isot., 2009, 67, 1162 CrossRef CAS PubMed.
- R. Chakravarty and A. Dash, J. Nanosci. Nanotechnol., 2013, 13, 243 CrossRef PubMed.
- R. Chakravarty and A. Dash, J. Radioanal. Nucl. Chem., 2014, 299, 741 CrossRef CAS PubMed.
- R. Chakravarty, R. Shukla, A. K. Tyagi and A. Dash, in Manipulation of nanoscale materials: An introduction to nanoarchitectonics, ed. K. Ariga, Royal Society of Chemistry, London, 2012, p. 259 Search PubMed.
- R. Chakravarty, R. Shukla, S. Gandhi, R. Ram, A. Dash, M. Venkatesh and A. K. Tyagi, J. Nanosci. Nanotechnol., 2008, 8, 4447 CrossRef CAS PubMed.
- R. Chakravarty, R. Shukla, R. Ram, A. K. Tyagi, A. Dash and M. Venkatesh, Chromatographia, 2010, 72, 875 CAS.
- R. Chakravarty, R. Ram, A. Dash and M. R. A. Pillai, Nucl. Med. Biol., 2012, 39, 916 CrossRef CAS PubMed.
- R. Chakravarty, R. Ram, D. Sen, S. Mazumder, M. R. A. Pillai and A. Dash, Ind. Eng. Chem. Res., 2013, 52, 11673 CrossRef CAS.
- R. Chakravarty, R. Ram and A. Dash, Sep. Sci. Technol., 2014, 49, 1825 CrossRef CAS.
- R. Chakravarty, R. Shukla, R. Ram, M. Venkatesh, A. Dash and A. K. Tyagi, ACS Appl. Mater. Interfaces, 2010, 2, 2069 CAS.
- R. Chakravarty, R. Shukla, R. Ram, A. K. Tyagi, A. Dash and M. Venkatesh, Nucl. Med. Biol., 2011, 38, 575 CrossRef CAS PubMed.
- R. Chakravarty, S. Chakraborty, R. Ram, A. Dash and M. R. A. Pillai, Cancer Biother. Radiopharm., 2013, 28, 631 CrossRef CAS PubMed.
- R. Chakravarty, A. Dash and M. Venkatesh, Chromatographia, 2009, 69, 1363 CAS.
- R. Chakravarty, R. Shukla, R. Ram, A. K. Tyagi, A. Dash and M. Venkatesh, Appl. Radiat. Isot., 2010, 68, 229 CrossRef CAS PubMed.
- R. Chakravarty, R. Shukla, R. Ram, M. Venkatesh, A. K. Tyagi and A. Dash, Anal. Chem., 2011, 83, 6342 CrossRef CAS PubMed.
- R. Chakravarty and A. Dash, Sep. Sci. Technol., 2013, 48, 607 CrossRef CAS.
- T. J. Morley, M. Dodd, K. Gagnon, V. Hanemaayer, J. Wilson, S. A. McQuarrie, W. English, T. J. Ruth, F. Bénard and P. Schaffer, Nucl. Med. Biol., 2012, 39, 551 CrossRef CAS PubMed.
- D. R. McAlister and E. P. Horwitz, Automated two column generator systems for medical radionuclides, Appl. Radiat. Isot., 2009, 67, 1985 CrossRef CAS PubMed.
- V. Romanovski, D. Wester, S. Bartenev, M. Zykov, G. Kuznetsov, L. Shlkjar, G. Kodina, S. Erofeev, N. Usacheva, V. Buntsev and E. Kolobokov, Separation of tungsten and rhenium on alumina, Extended synopses from the third Russian-Japanese seminar on technetium, Dubna, Russia, June 23–July 1, 2002,p. 14 Search PubMed.
- D. A. Tkachuk and M. P. Zykov, Eur. J. Nucl. Med. Mol. Imaging, 2006, 33(suppl. 2), 384 Search PubMed.
- On the industrial production of high purity 90Y, 99mTc and 188Re radionuclides for medical purposes using centrifugal semi countercurrent extraction generators, http://eng.phyche.ac.ru/?p=4277.
- S. Mirzadeh, M. Du, A. Beets and F. F. Knapp Jr, Ind. Eng. Chem. Res., 2000, 39, 3169 CrossRef CAS.
- A. F. Novgorodov, F. Bruchertseifer, J. Brockmann, N. A. Lebedev and F. Rösch, Radiochim. Acta, 2000, 88, 163 CrossRef CAS PubMed.
- F. Bruchertseifer, J. Brockmann, A. F. Novgorodov, N. A. Lebedev and F. Rösch, J. Labelled Compd. Radiopharm., 1999, 42(suppl 1), 930 Search PubMed.
- A. F. Novgorodov, F. Rösch and N. A. Korolev, Radiochemical separations by thermochromatography, in Handbook of Nuclear Chemistry, ed. A. Vértes, S. Nagy, Z. Klencsár, R. G. Lovas and F. Rösch, 2011, p. 2429 Search PubMed.
- A. Dash and R. Chakravarty, Ind. Eng. Chem. Res., 2014, 53, 3766 CrossRef CAS.
- R. Chakravarty, A. Dash and M. R. A. Pillai, Curr. Radiopharm., 2012, 5, 271 CrossRef CAS.
- R. Chakravarty, S. Chakraborty, V. Chirayil and A. Dash, Nucl. Med. Biol., 2014, 41, 163 CrossRef CAS PubMed.
- R. Chakravarty, M. Venkatesh and A. Dash, J. Radioanal. Nucl. Chem., 2011, 290, 45 CrossRef CAS.
- R. Chakravarty, T. Das, A. Dash and M. Venkatesh, Nucl. Med. Biol., 2010, 37, 811 CrossRef CAS PubMed.
- R. Chakravarty, T. Das, M. Venkatesh and A. Dash, Radiochim. Acta, 2012, 100, 255 CrossRef CAS.
- R. Chakravarty, A. Dash and M. Venkatesh, Nucl. Med. Biol., 2010, 37, 2 Search PubMed.
- R. Chakravarty, U. Pandey, R. B. Manolkar, A. Dash, M. Venkatesh and M. R. A. Pillai, Nucl. Med. Biol., 2008, 35, 245 CrossRef CAS PubMed.
- R. Chakravarty, A. Dash, M. R. A. Pillai and M. Venkatesh, Radiochim. Acta, 2009, 97, 309 CrossRef CAS.
- Automated electrochemical 90Y generator, http://www.elexcomm.com/kamadhenu-eng.html.
- EudraLex—the rules governing medicinal products in the European Union, EU guidelines to good manufacturing practice medicinal products for human and veterinary use. Part II: basic requirements for active substances used as starting materials, October 2005, vol. 4, http://ec.europa.eu/enterprise/pharmaceuticals/eudralex/vol-4/pdfs-en/2005_0_03_gmp-partii-activesubstance.pdf Search PubMed.
- N. Brief, ORNL Implements cGMP for 188Re Generators, J. Nucl. Med., 2010, 51, 14N Search PubMed , http://jnm.snmjournals.org/content/51/1/14N.ull.pdf.
- R. J. Callahan, Semin. Nucl. Med., 1996, 26, 85 CrossRef CAS.
- G. B. Saha, Fundamentals of Nuclear Pharmacy, Publishers-Springer Verlag, New York, 6th edn, 2010 Search PubMed.
- R. De Arellano, C. Piera, J. Pavia and J. Setoain, Nucl. Med. Commun., 1999, 20, 279 CrossRef PubMed.
- A. T. Elliot, T. E. Hilditch, T. Murray and H. McNutty, Nucl. Med. Commun., 1993, 14, 328 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2015 |