Highly conductive polymer composites incorporated with electrochemically exfoliated graphene fillers†
Abstract
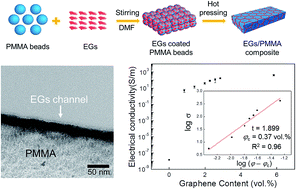
Highly conductive polymer composites were fabricated with a novel type of graphene-based filler, electrochemically exfoliated graphene sheets (EGs) produced without conventional oxidation/reduction of graphene oxide (GO). The high electrical conductivity of 417 ± 83 S m−1 at a content loading of 5.8 vol% and a low percolation threshold of 0.37 vol% could be attainable. Microscopic and spectroscopic investigations demonstrated the effective dispersion of EG-fillers on the surface of polymer particles and visualized the actual conducting channel between the polymer matrices.

 Please wait while we load your content...
Please wait while we load your content...