NIS-mediated intramolecular oxidative α-functionalization of tertiary amines: transition metal-free synthesis of 1,2-dihydro-(4H)-3,1-benzoxazin-4-one derivatives†
Abstract
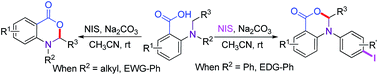
A novel method for direct α-functionalization of tertiary amines via NIS-mediated oxidative C–O bond formation, where NIS serves as both an oxidant and an iodination reagent, has been developed. The method provides easy access to either iodinated or non-iodinated 1,2-dihydro-(4H)-3,1-benzoxazin-4-ones, depending on the nature of the reactant, via a regioselective transition metal-free approach.

 Please wait while we load your content...
Please wait while we load your content...