Simple electrochemical synthesis of ultra-long silver telluride nanotubes†
Abstract
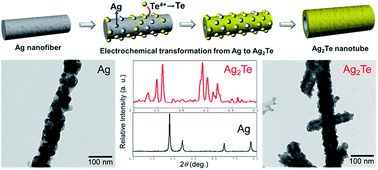
A simple synthetic route for ultra-long silver telluride (Ag2Te) nanotubes is presented. Silver (Ag) nanofibers electro-spun on a plastic substrate could be electrochemically transformed into Ag2Te nanotubes. The electrochemical transformation proceeded through the electrodeposition of tellurium (Te) on the surface of Ag nanofibers, which induces the Ag atoms to diffuse into the layers of Te, and thus leads to a structural and morphological change from nanofibers to hollow nanotubes.

 Please wait while we load your content...
Please wait while we load your content...