Direct trifluoromethylation of imidazoheterocycles in a recyclable medium at room temperature†
Abstract
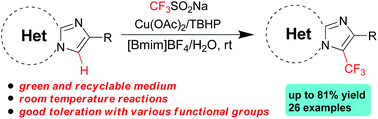
Regioselective C–H trifluoromethylation of imidazoheterocycles with Langlois' reagent in a recyclable mixed medium of 1-butyl-3-methylimidazoliumtetrafluoroborate ([Bmim]BF4) and water at room temperature has been developed. In the presence of catalytic cupric acetate and 2-methyl-2-(methylperoxy)propane (TBHP), this green strategy tolerates a wide range of functional groups to afford diverse trifluoromethylated imidazoheterocycles in moderate to good yields.

 Please wait while we load your content...
Please wait while we load your content...