Preparation of surface self-concentration and contact-killing antibacterial coating through UV curing†
Abstract
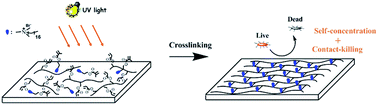
A facile method to prepare surface self-concentration antibacterial coating with contact-killing mechanism was developed. Photo curable quaternary ammonium compound QAC bearing a 16-carbon alkyl chain and a terminal methacrylate was synthesized as reactive antibacterial additive. After UV irradiation, QAC was crosslinked in the soy-based coating matrix. With proper amounts of QAC, the UV cured coatings exhibited good properties such as high glossiness, good hardness and fine adhesion to cherrywood substrates. The self-concentration property of QAC on the cured coating surface, which is beneficial to reserve the physical properties of the bulk materials and to improve the surface antibacterial activity, was confirmed by fluorescence and X-ray photoelectron spectroscopy. Moreover, the introduction of QAC provided the coatings with superhydrophilicity property as well as good anti-fog capacity. The coatings with 8 wt% of QAC (charge density = 8.6 mol cm−2) exhibited almost 100% antibacterial activity against Gram-positive Staphylococcus aureus and Gram-negative Escherichia coli by 5 log reduction. The contact-killing mechanism of coatings was confirmed via the zone of inhibition tests. The straightforward preparation combined with excellent antibacterial property makes the QAC containing coatings quite promising in antibacterial coatings realm and is suitable for industrial-scale manufacturing.

 Please wait while we load your content...
Please wait while we load your content...