Degradation of cationic and anionic dyes in coagulation–flocculation process using bi-functionalized silica hybrid with aluminum-ferric as auxiliary agent
Abstract
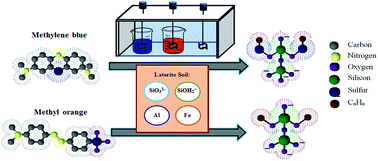
Cationic dye (methylene blue) and anionic dye (methyl orange) degradation in the coagulation process was demonstrated. The key material was a natural coagulant–laterite soil dominated by a silica component, while aluminum-ferric ions acted as an auxiliary agent in the degradation process. Charge neutralization, electrical double layer compression and sweeping flocculation were the mechanisms in the decolorization reaction. These results provided a new insight into effective dye degradation using a new class of natural coagulant–natural resources (laterite soil). The complex molecular structure of methylene blue and methyl orange was degraded into smaller hydrocarbon forms, accompanied by the formation of silsesquioxane. The silsesquioxane was the final product of degradation with promising flocculation and low volume sludge. Lastly, a comparison of the aluminum-based coagulant and a laterite soil natural coagulant shows a clear vision of the performance for both types of coagulant.

 Please wait while we load your content...
Please wait while we load your content...