Magnetic iron oxide and iron oxide@gold nanoparticle anchored nitrogen and sulfur-functionalized reduced graphene oxide electrocatalyst for methanol oxidation
Abstract
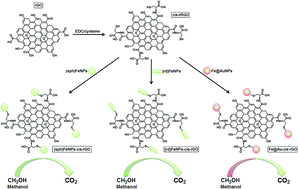
Fuel cells have been attracting more and more attention in recent decades due to high-energy demands, fossil fuel depletions and environmental pollution throughout world. In this study, we report the synthesis of metallic and bimetallic nanoparticles such as spherical iron oxide nanoparticles [(sp)Fe3O4], rod iron oxide nanoparticles [(rd)Fe3O4] and iron@gold nanoparticles (Fe3O4@AuNPs) involving L-cysteine functionalized reduced graphene oxide nanohybrids [(sp)Fe3O4/cys/rGO, (rd)Fe3O4/cys/rGO and Fe3O4@AuNPs/cys/rGO] and their application as an electrocatalyst for methanol electro-oxidation. The nanohybrids have been characterized by transmission electron microscopy (TEM), X-ray photoelectron spectroscopy (XPS) and X-ray diffraction (XRD). The experimental results have demonstrated that reduced graphene oxide-supported bimetallic nanoparticles enhanced the electrochemical efficiency for methanol electro-oxidation with regard to diffusion efficiency, oxidation potential and forward oxidation peak current. Fe3O4@AuNPs/cys/rGO, in comparison to (sp)Fe3O4/cys/rGO and (rd)Fe3O4/cys/rGO, showed the most efficiency for methanol electro-oxidation.

 Please wait while we load your content...
Please wait while we load your content...