Entrapment of radioactive uranium from wastewater by using fungus-Fe3O4 bio-nanocomposites†
Abstract
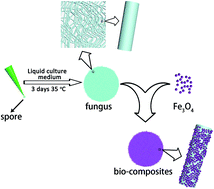
Magnetically separable adsorbents with high sorption capacity for nuclear wastewater treatment have been successfully synthesized on the basis of fungus-Fe3O4 nanoparticle bio-nanocomposites through a simple co-culture method. Experiments demonstrated that fungus-functionalized Fe3O4 nanoparticles can be beneficial for avoiding the phenomenon of aggregation for Fe3O4 nanoparticles and effectively separating the adsorbents from the contaminated water after adsorption without sacrificing the performances of magnetic adsorbent Fe3O4. Indeed, the bio-nanocomposites with improved sorption capacity can be attributed to the quasi-monodisperse Fe3O4 nanomaterials uniformly grown on the macro-scale fungus body. Furthermore, the promising adsorbents with high biological activity can effectively and synergistically remove the radioactive ions from nuclear wastewater with the saturated sorption capacity up to 171 mg g−1 for (UO2)2+ ions. The fast adsorption process can guarantee that the polluted water could be recovered within only four hours. Our results clearly indicate that the bio-nanocomposites could be used as cost-effective radioactive adsorbents.

 Please wait while we load your content...
Please wait while we load your content...